The chemical element hydrogen is classed as a nonmetal. It can become metallic at very high pressures. It was discovered in 1766 by Henry Cavendish.

Data Zone
| Classification | Hydrogen is a nonmetal. It can become metallic at very high pressures. |
| Color | colorless |
| Atomic weight | 1.0079 |
| State | gas |
| Melting point | -259.14 oC, 14.01 K |
| Boiling point | -252.87 oC, 20.28 K |
| Electrons: | 1 |
| Protons: | 1 |
| Neutrons in most abundant isotope: | 0 |
| Electron shells | 1 |
| Electron configuration | 1s1 |
| Density @ 20oC | 0.0000899 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume | 14.4 cm3/mol |
| Structure | hcp: hexagonal close packed (as solid at low temperatures) |
| Hardness | – |
| Specific heat capacity | 14.304 J g-1 K-1 |
| Heat of fusion | 0.117 kJ mol-1 of H2 |
| Heat of atomization | 218 kJ mol-1 |
| Heat of vaporization | 0.904 kJ mol-1 of H2 |
| 1st ionization energy | 1312 kJ mol-1 |
| 2nd ionization energy | kJ mol-1 |
| 3rd ionization energy | 11815.0 kJ mol-1 |
| Electron affinity | 72.7711 kJ mol-1 |
| Minimum oxidation number | -1 |
| Min. common oxidation no. | -1 |
| Maximum oxidation number | 1 |
| Max. common oxidation no. | 1 |
| Electronegativity (Pauling Scale) | 2.18 |
| Polarizability volume | 0.7 Å3 |
| Reaction with air | vigorous, ⇒ H2O |
| Reaction with 15 M HNO3 | none |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | H2O |
| Hydride(s) | H2 |
| Chloride(s) | HCl |
| Atomic radius | 25 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 0.1805 W m-1 K-1 |
| Electrical conductivity | – |
| Freezing/Melting point: | -259.14 oC, 14.01 K |

Nasa image: Vast quantities of hydrogen in remote galaxies.

Theophrastus Paracelsus – The first person to generate hydrogen: "Air arises and breaks forth like a wind."
Discovery of Hydrogen
A favorite school chemistry experiment is to add a metal such as magnesium to an acid. The metal reacts with the acid, forming a salt and releases hydrogen from the acid. The hydrogen gas bubbles up from the liquid and students collect it in small quantities for further experiments, such as the ‘pop-test.’
The first recorded instance of hydrogen made by human action was in the first half of the 1500s, by a similar method to that used in schools now. Theophrastus Paracelsus, a physician, dissolved iron in sulfuric acid and observed the release of a gas. He is reported to have said of the experiment, “Air arises and breaks forth like a wind.” He did not, however, discover any of hydrogen’s properties.(1)
Turquet De Mayerne repeated Paracelsus’s experiment in 1650 and found that the gas was flammable.(2) Neither Paracelsus nor De Mayerne proposed that hydrogen could be a new element. Indeed, Paracelsus believed there were only three elements – the tria prima – salt, sulfur, and mercury – and that all other substances were made of different combinations of these three. (3) (Chemistry still had a long way to go!)
In 1670, English scientist Robert Boyle added iron to sulfuric acid. He showed the resulting (hydrogen) gas only burned if air was present and that a fraction of the air (we would now call it oxygen) was consumed by the burning.(4)
Hydrogen was first recognized as a distinct element in 1766 by English scientist Henry Cavendish, when he prepared it by reacting hydrochloric acid with zinc. He described hydrogen as “inflammable air from metals” and established that it was the same material (by its reactions and its density) regardless of which metal and which acid he used to produce it.(1) Cavendish also observed that when the substance was burned, it produced water.
French scientist Antoine Lavoisier later named the element hydrogen (1783). The name comes from the Greek ‘hydro’ meaning water and ‘genes’ meaning forming – hydrogen is one of the two water forming elements.
In 1806, with hydrogen well-established as an element, English chemist Humphry Davy pushed a strong electric current through purified water.
He found hydrogen and oxygen were formed. The experiment demonstrated that electricity could pull substances apart into their constituent elements. Davy realized that substances were bound together by an electrical phenomenon; he had discovered the true nature of chemical bonding.(5)
Visit Chemicool’s Cool Hydrogen Facts Page.

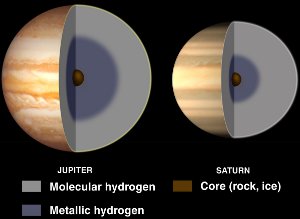
Interiors of Jupiter and Saturn, with liquid metallic hydrogen. Courtesy NASA/JPL-Caltech.

Nasa: The Space Shuttle’s external fuel tank (orange) filled with liquid hydrogen and oxygen.

Hydrogen cars emit water rather than pollutants.

Laboratory electrolysis of water. Electrical energy is used to split water. Hydrogen gathers in one test-tube, oxygen in the other.
Appearance and Characteristics
Harmful effects:
Hydrogen is highly flammable and has an almost invisible flame, which can lead to accidental burns.
Characteristics:
Hydrogen is the simplest element of all, and the lightest. It is also by far the most common element in the Universe. Over 90 percent of the atoms in the Universe are hydrogen.
In its commonest form, the hydrogen atom is made of one proton, one electron, and no neutrons. Hydrogen is the only element that can exist without neutrons.
Hydrogen is a colorless, odorless gas which exists, at standard temperature and pressure, as diatomic molecules, H2.
It burns and forms explosive mixtures in air and it reacts violently with oxidants.
On Earth, the major location of hydrogen is in water, H2O. There is little free hydrogen on Earth because hydrogen is so light that it is not held by the planet’s gravity. Any hydrogen that forms eventually escapes from the atmosphere into space.
Although hydrogen is usually a nonmetal, it becomes a liquid metal when enormous pressures are applied to it.
Such pressures are found within gas giant planets such as Jupiter and Saturn. Jupiter’s high magnetic field (14 times Earth’s) is believed to be caused by a dynamo effect resulting from electrically conducting metallic hydrogen circulating as the planet rotates.
Uses of Hydrogen
Large quantities of hydrogen are used in the Haber process (production of ammonia), hydrogenation of fats and oils, methanol production, hydrocracking, and hydrodesulfurization. Hydrogen is also used in metal refining.
Liquid hydrogen is used as a rocket fuel, for example powering the Space Shuttle’s lift-off and ascent into orbit. Liquid hydrogen and oxygen are held in the Shuttle’s large, external fuel tank. (See image left.)
Hydrogen’s two heavier isotopes (deuterium and tritium) are used in nuclear fusion.
The hydrogen economy has been proposed as a replacement for our current hydrocarbon (oil, gas and coal) based economy.
The basis of the hydrogen economy is that energy is produced when hydrogen combusts with oxygen and the only by-product from the reaction is water.
At the moment, however, the hydrogen for hydrogen-powered cars is produced from hydrocarbons. Only when solar or wind energies, for example, can be used commercially to split water into hydrogen and oxygen will a true hydrogen economy be possible.
Abundance and Isotopes
Abundance earth’s crust: 1400 parts per million by weight (0.14%), 2.9% by moles
Abundance solar system: 75% by weight, 93% by moles
Cost, pure: $12 per 100g
Cost, bulk: $ per 100g
Source: Hydrogen is prepared commercially by reacting superheated steam with methane or carbon. In the laboratory, hydrogen can be produced by the action of acids on metals such as zinc or magnesium, or by the electrolysis of water (shown on the left).
Isotopes: Hydrogen has three isotopes, 1H (protium), 2H (deuterium) and 3H (tritium). Its two heavier isotopes (deuterium and tritium) are used for nuclear fusion. Protium is the most abundant isotope, and tritium the least abundant. Tritium is unstable with a half-life of about 12 years 4 months. Naturally occurring hydrogen is a mixture of the two isotopes 1H and 2H with natural abundances of 99.99% and 0.01% respectively.

References
- Peter Hoffmann, Tomorrow’s Energy: Hydrogen, Fuel Cells, and the Prospects for a Cleaner Planet., (2001) p22. MIT Press, Cambridge, MA.
- P. Litherland Teed, The Chemistry and Manufacture of Hydrogen., (2008) p2. Dabney Press.
- John S. Davidson, Annotations to Boyle’s “The Sceptical Chymist”.
- Andreas Züttel, Andreas Borgschulte, Louis Schlapbach, Hydrogen as a future energy carrier., (2008) p8. Wiley-VCH, Weinheim.
- Kendall Haven, 100 Greatest Science Discoveries of All Time., (2007) p62. Libraries Unlimited.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/hydrogen.html">Hydrogen</a>
or
<a href="https://www.chemicool.com/elements/hydrogen.html">Hydrogen Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Hydrogen." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web. <https://www.chemicool.com/elements/hydrogen.html>.
I had read that when hydrogen reaches the point of saturation, it turns green; also, isn’t hydrogen one of the atmospheric gases?
Sorry, I don’t know what you mean by the point of saturation, but I’ve never heard of hydrogen turning green! There are tiny amounts of hydrogen in the atmosphere, but it’s always being lost to space, because earth’s gravity isn’t strong enough to hold on to such a light molecule.
Hydrogen can not exist in the earth’s atmosphere because it’s light weight and it’s large atom speed. It escapes easily to the outer space.
Now about the Hindenberg… All those flame that you see are clearly NOT hydrogen. How can you know this? Well, because you can SEE them. Besides hydrogen “fires” tend to be explosions because the reaction with oxygen is so simple and fast. To have a sustained hydrogen fire you have to contain and meter the H2 supply to keep a flammable ratio of H2 and O2 (naturally pure H2 doesn’t burn). The gas quickly disperses because of its high thermal velocity and could never have stuck around as long as the video. The Hindenberg had many other problems besides the H2 in it, such as explosively flammable reflective paint. I doubt that helium would have prevented those bright orange flames any better than hydrogen.
Is it not possible (perhaps even likely) that hydrogen was involved in the initial and very early stages.
Is it also not possible that if the fire started and the airship used helium instead of hyrogen that the release of helium could have strarved the flames of a significant amount of oxygen
In both cases helium instead of hydrogen would have been significant
I love the facts it helped me in school. Thanks Dr. Stewart!!!!!!!!!!!!!!!!!!
i love this website it helps me soooo much with science homework and projects there is soo much things i have learned fromm this website .it is soooo interesting especially when your favorite subject is science this website rocks!
this is the best website i have been to
This really helped me a lot for a school project and i am very thankful for it!!!!!!!!!!!! 🙂
i love this website! its so easy and conveinent! i can find my info. for science projects without having to search all over the internet!
It was really helpful thank you……………………….