The chemical element beryllium is classed as an an alkali earth metal. Pure beryllium was discovered in 1828 by Friederich Wöhler and Antoine Bussy.

Data Zone
| Classification: | Beryllium is an alkali earth metal |
| Color: | steel gray |
| Atomic weight: | 9.01218 |
| State: | solid |
| Melting point: | 1278 oC, 1551.2 K |
| Boiling point: | 2469 oC, 2742 K |
| Electrons: | 4 |
| Protons: | 4 |
| Neutrons in most abundant isotope: | 5 |
| Electron shells: | 2,2 |
| Electron configuration: | 1s2 2s2 |
| Density @ 20oC: | 1.848 g/cm3 |
Compounds, Radii, Conductivities
| Atomic volume: | 4.9 cm3/mol |
| Structure: | hcp: hexagonal close packed |
| Hardness: | 5.5 mohs |
| Specific heat capacity | 1.82 J g-1 K-1 |
| Heat of fusion | 7.895 kJ mol-1 |
| Heat of atomization | 324 kJ mol-1 |
| Heat of vaporization | 297 kJ mol-1 |
| 1st ionization energy | 899.5 kJ mol-1 |
| 2nd ionization energy | 1757.1 kJ mol-1 |
| 3rd ionization energy | 14848.7 kJ mol-1 |
| Electron affinity | 0 kJ mol-1 |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 2 |
| Max. common oxidation no. | 2 |
| Electronegativity (Pauling Scale) | 1.57 |
| Polarizability volume | 5.6 Å3 |
| Reaction with air | vigourous, w/ht ⇒ BeO, Be3N2 |
| Reaction with 15 M HNO3 | none |
| Reaction with 6 M HCl | mild ⇒ H2 |
| Reaction with 6 M NaOH | mild ⇒ H2, [Be(OH)4]2 |
| Oxide(s) | BeO3 |
| Hydride(s) | BeH2 |
| Chloride(s) | BeCl2 |
| Atomic radius | 112 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | 45 pm |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 200 W m-1 K-1 |
| Electrical conductivity | 25 x 106 S m-1 |
| Freezing/Melting point: | 1278 oC, 1551.2 K |

Beryllium Foils. Image: Deglr6328 (Ref. 6.)

Three varieties of beryl (left) and an emerald (right). Beryl and emerald’s formula is Be3Al2(SiO3)6. The different colors are caused by traces of different elements. For example, emeralds are colored by traces of chromium 3+ (blue-green) or vanadium 3+ (yellow-green) ions. (5) Images from Reno Chris and Jan Arkesteijn.
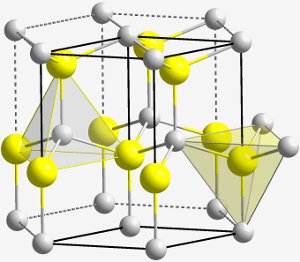
The hexagonal crystal structure of BeO (beryllium oxide). Study of crystals of beryl and emerald provided a clue to the existence of the new element beryllium. Image from Solid State
Discovery of Beryllium
In 1798, in France, René Haüy saw similarities in the crystal structures and properties of beryl and emerald. Beryl can appear in a number of different colors. Emerald is green. (See images on left.)
Haüy wondered if, despite their different colors, beryl and emerald could be made of the same elements. He approached Nicolas Louis Vauquelin, a French chemist who specialized in analysis, and asked him to have a look. (1)
Vauquelin discovered a new, sweet-tasting substance in both emerald and beryl. We now call this substance beryllia, BeO. Despite its sweet taste, we now know that beryllium and its compounds are highly toxic.
Although frowned upon today, old style chemists often tasted chemicals as part of their analyses.
For some, the taste test put their careers into terminal decline. One such chemist was Karl Scheele from Sweden, who discovered chlorine and oxygen. Scheele is believed to have died from poisoning caused by a variety of his experiments.
Vauquelin proposed that beryllia contained a previously undiscovered element, an earth metal. He initially called this new element ‘earth of beryl.’ (2)
The sweet taste of the salts then led to the new element being renamed ‘glyceynum,’ then ‘glucina’ or ‘glucine.’ The Greek ‘glykis’ means ‘sweet’ and is the source of our word ‘glucose.’ (1), (3)
Pure beryllium was first isolated from its salts in 1828 by Friederich Wöhler in Germany and, independently, Antoine Bussy in France.
Both chemists reacted potassium with beryllium chloride in a platinum crucible yielding potassium chloride and beryllium.
Wöhler was unhappy with the name the new element had been given, preferring beryllium from the Greek word ‘beryllos,’ meaning the mineral beryl.
Wöhler’s countryman, Martin Klaproth, had already pointed out in 1801 that yttria also forms sweet salts. A name derived from ‘beryllos’ would be less likely to cause confusion than one derived from ‘glykis.’ Klaproth also noted that a genus of plants was already called glucine. (4)
Bussy, however, preferred to call the new element ‘glucinium.’
Finally, in 1949, IUPAC chose beryllium as the element’s name and this decision became official in 1957. (2)
Beryllium played a large part in proving the existence of neutrons. In 1932, James Chadwick, an English physicist, bombarded a sample of beryllium with alpha-rays (helium nuclei). He observed that the bombarded sample emitted a subatomic particle, which had mass but no charge.
This neutral particle was the neutron.


A large beryllium crystal of 99%+ purity. (Photo: Alchemist-hp)
Appearance and Characteristics
Harmful effects:
Beryllium and its salts are both toxic and carcinogenic.
Characteristics:
Beryllium is light, silver-gray, relatively soft metal that is strong but brittle.
Beryllium has the highest melting point of the light metals, melting at 1278 oC – considerably higher than, for example, Lithium (180 oC) Sodium (98 oC) Magnesium (650 oC) Aluminum (660 oC) or Calcium (839 oC).
Under normal conditions, a thin layer of the hard oxide BeO forms on beryllium’s surface, protecting the metal from further attack by water or air.
As a result of this BeO layer, beryllium does not oxidize in air even at 600oC and it resists corrosion by concentrated nitric acid.
Beryllium also has high thermal conductivity and is nonmagnetic
Uses of Beryllium
Unlike most metals, beryllium is virtually transparent to x-rays and hence it is used in radiation windows for x-ray tubes.
Beryllium alloys are used in the aerospace industry as light-weight materials for high performance aircraft, satellites and spacecraft.
Beryllium is used as an alloy with copper to make spark-proof tools.
Beryllium is also used in nuclear reactors as a reflector and absorber of neutrons, a shield and a moderator.
Abundance and Isotopes
Abundance earth’s crust: 2.8 parts per million by weight, 4.6 parts per million by moles
Abundance solar system: parts per billion by weight, parts per billion by moles
Cost, pure: $748 per 100g
Cost, bulk: $93 per 100g
Source: The mineral beryl, Be3Al2(SiO3)6 is the most important source of beryllium.
Commercially it is produced by the reduction of the fluoride with magnesium metal.
Isotopes: Beryllium has nine isotopes with known half-lives. 9Be is the only stable isotope.
Cosmogenic 10Be (half-life 1.51 million years) is produced in the atmosphere by the impact of cosmic rays on oxygen and nitrogen.

References
- Edward Smedley, Hugh James Rose, Henry John Rose, Encyclopaedia Metropolitana; or Universal Dictionary of Knowledge., 1845, Volume 4, p693 William Clowes and Sons.
- Kenneth A. Walsh, Beryllium Chemistry and Processing., 2009, p7, ASM International.
- Mary Elvira Weeks, The Discovery of the Elements. XII., J. Chem. Educ., 1932, 9 (8), p1386
- Martin Heinrich Klaproth, Analytical Essays Towards Promoting the Chemical Knowledge of Mineral Substances., 1801, LXXVI, p59, T. Cadell, Jun. and W. Davies.
- George Robert Rapp, Archaeomineralogy., 2002, p101, Springer.
- Image courtesy Deglr6328; GNU.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/beryllium.html">Beryllium</a>
or
<a href="https://www.chemicool.com/elements/beryllium.html">Beryllium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Beryllium." Chemicool Periodic Table. Chemicool.com. 15 Oct. 2012. Web. <https://www.chemicool.com/elements/beryllium.html>.
thank you so much i have a project i am doing for 8th grade science and this is helping me so much i appreciate all your hard work. 🙂
This helped me for my element project for Pre-AP Physical Science/9th Grade
what is the atomic radius
Hi Justin, It’s in the Data Zone at the top of the page. Atomic radius 112 pm (picometers).
This helped me a lot for my Pre-AP Chemistry project! I had no idea that Nicolas Louis Vaquelin didn’t actually find that Beryl and Emerald had something in common. I had alway thought he was the one who found the similarities and study it from there!
Oh and what year did Nicolas Louis Vaquelin end his research?
Hi Maddie, I’m glad it was helpful. The research that Vauquelin carried out that’s reported here ended in 1798. I don’t know if Vauquelin did any further work with beryllium following his discovery of ‘earth of beryl.’
This helps so much!!! I’m doing a 6th grade elemental study and this really helps