The chemical element ytterbium is classed as a lanthanide and rare earth metal. It was discovered in 1878 by Jean Charles Galissard de Marignac.

Data Zone
| Classification: | Ytterbium is a lanthanide and rare earth metal |
| Color: | silvery-white |
| Atomic weight: | 173.04 |
| State: | solid |
| Melting point: | 824 oC, 1097 K |
| Boiling point: | 1200 oC, 1473 K |
| Electrons: | 70 |
| Protons: | 70 |
| Neutrons in most abundant isotope: | 104 |
| Electron shells: | 2,8,18,32,8,2 |
| Electron configuration: | [Xe] 4f14 6s2 |
| Density @ 20oC: | 6.97 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 24.79 cm3/mol |
| Structure: | face-centered cubic |
| Specific heat capacity | 0.15 J g-1 K-1 |
| Heat of fusion | 7.66 kJ mol-1 |
| Heat of atomization | 152 kJ mol-1 |
| Heat of vaporization | 128.90 kJ mol-1 |
| 1st ionization energy | 603.4 kJ mol-1 |
| 2nd ionization energy | 1176 kJ mol-1 |
| 3rd ionization energy | 2415 kJ mol-1 |
| Electron affinity | – |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 3 |
| Max. common oxidation no. | 3 |
| Electronegativity (Pauling Scale) | 1.1 |
| Polarizability volume | 21.0 Å3 |
| Reaction with air | mild, ⇒ Yb2O3 |
| Reaction with 15 M HNO3 | mild, ⇒ Yb(NO3)3 |
| Reaction with 6 M HCl | mild, ⇒ H2, YbCl3 |
| Reaction with 6 M NaOH | – |
| Oxide(s) | Yb2O3 (ytterbia) |
| Hydride(s) | YbH2, Yb2H5 |
| Chloride(s) | YbCl2, YbCl3 |
| Atomic radius | 175 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 116 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 38.5 W m-1 K-1 |
| Electrical conductivity | 3.7 x 106 S m-1 |
| Freezing/Melting point: | 824 oC, 1097 K |
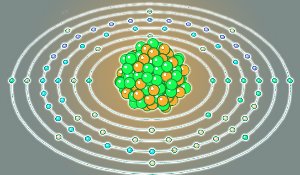
Ytterbium-174 is ytterbium’s commonest isotope, with 104 neutrons and 70 protons/electrons. Image Ref.(5)

This photo shows about 1 million ytterbium atoms illuminated by a blue laser in an experimental atomic clock that holds the atoms in a lattice made of intersecting laser beams. Scientists are studying the possible use of ytterbium atoms in next-generation atomic clocks based on optical frequencies, which could be more stable and accurate than today’s best time standards, which are based on microwave frequencies. Photo: Barber, NIST.
Discovery of Ytterbium
Ytterbium was discovered by Jean Charles Galissard de Marignac in 1878, in Geneva, Switzerland.
He heated erbium nitrate until it decomposed and then extracted the residue, which contained an unknown white powder that he named ytterbium oxide (ytterbia). (1), (2)
In 1907, in Paris, George Urbain separated ytterbia into two constituents. He performed a series of fractional crystallizations of ytterbium nitrate from nitric acid solution and obtained two rare earth oxides. One retained the name of ytterbium, the other he called lutetium. (1), (3)
Ytterbium metal was first made in 1937 by Klemm and Bonner by heating ytterbium chloride and potassium together. (2), (4)
Ytterbium pure metal was produced in 1953, at the Ames Laboratory, Iowa, by A. Daane, David Dennison and Frank Spedding from which the chemical and physical properties of the element could be measured. (2), (4)
The element is named after Ytterby, a village in Sweden. Four elements are named after this town, the others being yttrium, terbium, and erbium.
Appearance and Characteristics
Harmful effects:
Ytterbium is considered to be moderately toxic.
Characteristics:
Ytterbium is a bright, soft, silvery-white metal that is both ductile and malleable.
It is a one of the rare earth metals.
The metal tarnishes quickly in air and reacts slowly with water.
It dissolves rapidly in mineral acids.
Uses of Ytterbium
Isotope 160Yb is radioactive and is used in portable x-ray machines that need no electricity.
Under very high physical stress Ytterbium’s electrical resistance increases by an order of magnitude. It is therefore used in stress gauges to monitor ground deformations caused by earthquakes or underground explosions.
Ytterbium is used in alloys and is added to stainless steel to improve grain refinement and strength.
Ytterbium fiber laser amplifiers are used in marking and engraving.
Ytterbium compounds are also used as catalysts in the organic chemical industry.
Abundance and Isotopes
Abundance earth’s crust: 3 parts per million by weight, 0.3 parts per million by moles
Abundance solar system: 1 part per billion by weight, 10 parts per trillion by moles
Cost, pure: $1400 per 100g
Cost, bulk: $ per 100g
Source: Ytterbium is not found free in nature but is found in a number of minerals: mainly monazite, gadolinite euxenite and xenotime. Historically, isolation of the rare earth elements from each other has been difficult and expensive because their chemical properties are so similar. Ion exchange and solvent extraction techniques developed since the 1940’s have lowered the cost of production.
Isotopes: Ytterbium has 30 isotopes whose half-lives are known, with mass numbers 151 to 180. Naturally occurring ytterbium is a mixture of seven isotopes and they are found in the percentages shown: 168Yb (0.1%), 170Yb (3.0%), 171Yb (14.3%), 172Yb (21.8%), 173Yb (16.1%), 174Yb (31.8%) and 176Yb (12.8%). The most abundant isotope is 174Yb at 31.8%.

References
- Mary Elvira Weeks, The Discovery of the Elements XVI., Journal of Chemical Education., October 1932, p1761 and p1769.
- John Emsley, Nature’s building blocks: an A-Z guide to the elements., Oxford University Press, 2003, p493.
- Robert E. Krebs, The history and use of our earth’s chemical elements: a reference guide., JGreenwood Publishing Group, 2006, p302.
- David R. Lide, CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data., CRC Press, 1999, 4-41.
- Image LBL
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/ytterbium.html">Ytterbium</a>
or
<a href="https://www.chemicool.com/elements/ytterbium.html">Ytterbium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Ytterbium." Chemicool Periodic Table. Chemicool.com. 18 Oct. 2012. Web. <https://www.chemicool.com/elements/ytterbium.html>.