The chemical element plutonium is classed as an actinide metal. It was discovered in 1940 by Glenn Seaborg, Edwin McMillan, Joseph Kennedy, and Arthur Wahl.

Data Zone
| Classification: | Plutonium is an actinide metal |
| Color: | silvery-white |
| Atomic weight: | (244), no stable isotopes |
| State: | solid |
| Melting point: | 639.4 oC, 912.5 K |
| Boiling point: | 3230 oC, 3503 K |
| Electrons: | 94 |
| Protons: | 94 |
| Neutrons in most abundant isotope: | 150 |
| Electron shells: | 2,8,18,32,24,8,2 |
| Electron configuration: | [Rn] 5f6 7s2 |
| Density @ 20oC: | 19.8 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 12.32 cm3/mol |
| Structure: | fcc: face-centered cubic |
| Hardness: | |
| Specific heat capacity | 0.13 J g-1 K-1 |
| Heat of fusion | 2.840 kJ mol-1 |
| Heat of atomization | 352 kJ mol-1 |
| Heat of vaporization | 344.0 kJ mol-1 |
| 1st ionization energy | 585 kJ mol-1 |
| 2nd ionization energy | – |
| 3rd ionization energy | – |
| Electron affinity | – |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 7 |
| Max. common oxidation no. | 4 |
| Electronegativity (Pauling Scale) | 1.3 |
| Polarizability volume | 24.5 Å3 |
| Reaction with air | ⇒ PuO |
| Reaction with 15 M HNO3 | passivated |
| Reaction with 6 M HCl | – |
| Reaction with 6 M NaOH | – |
| Oxide(s) | PuO, Pu2O3, PuO2 |
| Hydride(s) | PuH2, PuH3 |
| Chloride(s) | PuCl2, PuCl3 |
| Atomic radius | 175 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 114 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 6.3 W m-1 K-1 |
| Electrical conductivity | 0.7 x 106 S m-1 |
| Freezing/Melting point: | 639.4 oC, 912.5 K |

A plutonium ‘button.’ Photo: Department of Energy
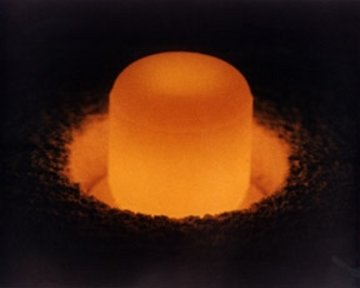
Plutonium-238 (plutonium oxide). Nuclear fission reactions release enough energy to increase the temperature of this sample of plutonium to red-heat. The heat produced by plutonium has been used as an energy source on spacecraft. Photo: Department of Energy.
Discovery of Plutonium
Plutonium was first produced in 1940 by Glenn Seaborg, Edwin McMillan, Joseph Kennedy, and Arthur Wahl. It was the second synthetic transuranium element of the actinide series to be discovered.
Plutonium-238 (half-life 87.7 years) was produced by deuteron bombardment of uranium-238 in the 60-inch cyclotron in Berkeley, California.
The Berkeley team made neptunium-238 (half-life 2.1 days) which decayed to plutonium-238: (1)
238U + 2H ⇒ 238Np +2n
ß decay: 238Np ⇒ 238Pu
The new element was identified chemically.
The metal was later found naturally in minute quantities as a decay product in uranium ores.
The much longer lived isotope plutonium-239 (half-life 24 110 years) was first made in 1941. Uranium-238 was bombarded with neutrons to produce uranium-239, which beta decayed to neptunium-239, which itself beta decayed to plutonium-239. (2)
In the same year it was found that slow neutrons cause plutonium-239 to undergo fission. The fission releases more neutrons, hence can result in a nuclear chain reaction. (See uranium for more about chain reactions.)
This discovery would lead to the use of plutonium as a source of nuclear energy. (2)
A microgram of pure plutonium-239 compound (plutonium IV iodate) was isolated in 1942 by Burris Cunningham and Louis Werner at the Metallurgical Laboratory of the University of Chicago.
This was the first time a compound of an artificially produced element had been made in a visible quantity, allowing detailed studies of its properties. (1), (2)
The metal was first isolated in 1943 by reducing plutonium trifluoride with lithium. A few small globules of silvery metal weighing 1-3 micrograms each were produced. (3)
The element is named after the planet Pluto, continuing the theme started by Martin Klaproth when he named uranium after the planet Uranus.


On Nov. 26, 2011 NASA launched ‘Curiosity’, the largest, most capable rover ever sent to another planet. Radioactive decay of 10.6 pounds (4.8 kilograms) of plutonium dioxide has produced a steady flow of heat to warm the rover’s systems during the intensely cold Martian night and allowed electricity to be generated. Image: NASA.

The Voyager 2 spacecraft, launched on Aug. 20, 1977, is about 17 billion kilometers (10 billion miles) from the sun. It is the longest continuously operating NASA spacecraft. It owes its long life to radioisotope thermoelectric generators; these generate electricity from heat flowing from plutonium-238’s radioactive decay. Image: NASA.
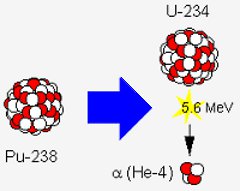
Radioactive decay of plutonium-238. Each decay produces uranium-234, an alpha-particle and a large amount of energy. Image: NASA.
Appearance and Characteristics
Harmful effects:
Plutonium is harmful due to its radioactivity.
Plutonium and its compounds are also toxic. It collects in the bones and the liver where it can remain for a long period of time. (4)
Characteristics:
Plutonium is a silvery radioactive metal that tarnishes in air to give a yellow oxide coating.
It has six allotropic forms, which vary widely in crystal structure and density.
The metal is chemically reactive, forming compounds with carbon, nitrogen, and silicon and the halogens.
Plutonium has five oxidation states (+3 to +7). These produce different colors in solution. For example, in 1 M perchlorate:
III: Pu3+ (blue lavender)
IV: Pu4+ (yellow brown)
V: PuO2+ (pink) (in sodium perchlorate)
VI: PuO22+(yellow)
VII: PuO52+ (olive green) (in sodium hydroxide). (5)
If you were to touch a small piece of plutonium metal (please don’t!) it would feel warm because of the energy released by alpha decay. A larger piece of the metal could boil water.
Uses of Plutonium
Plutonium-239, which can undergo nuclear chain reactions, is used in nuclear bombs and nuclear reactors
Plutonium-238 is used as a long-lived heat and power source for space probes. (Its intrinsic heat output is approximately 0.5 watts per gram.) The Pioneer and Voyager space probes used plutonium-238 nuclear batteries as a power source.
Three radioisotope heater units (each containing 2.7 grams of plutonium-238 dioxide) were used as heat sources on the Pathfinder Mars robot lander. Each radioisotope heater unit produces about one watt of heat. (6), (7)
Early pacemaker batteries also used tiny amounts of plutonium-238.
The image on the left shows the decay of one atom of plutonium-238. This releases 5.6 million electron volts of energy. To get an idea of what this means, consider NASA’s Curiosity Mars rover, which will be powered by 4.8 kg of plutonium dioxide.
During its first 87.7 year half-life, the plutonium will produce about 4800 gigajoules of energy. To generate the same energy using natural gas (mainly methane) the Mars rover would need to carry about 86 metric tons of methane and 345 metric tons of oxygen.
Abundance and Isotopes
Abundance earth’s crust: negligible
Abundance solar system: unknown
Cost, pure: $4000 per gram
Cost, bulk: per 100g
Source: Plutonium is found naturally in minute quantities in uranium ores. Commercially, it is produced in large quantities in nuclear reactors from 238U.
Isotopes: Plutonium has 17 whose half-lives are known, with mass numbers from 227 to 248. None are stable. Its longest lived isotopes are 244Pu, with a half-life of 80.8 million years,242Pu with a half-life of 373,300 years and 239Pu with a half-life of 24,110 years.

References
- I. Perlman, The Transuranium Elements and Nuclear Chemistry., Journal of Chemical Education., May 1948, p275.
- David L. Clark, Siegfried S. Hecker, Gordon D. Jarvinen,Mary P. Neu, The Chemistry of the Actinide and Transactinide Elements., Springer., Vol 2.10., p815.
- Los Alamos Science, Plutonium Metal. (pdf document)
- Argonne National Laboratory, Plutonium Human Health Fact Sheet. (pdf document)
- Los Alamos Science, The Chemical Complexities of Plutonium. (pdf document)
- Los Alamos Science, Plutonium in Use. (pdf document)
- NASA, Technologies for Severe Environments.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/plutonium.html">Plutonium</a>
or
<a href="https://www.chemicool.com/elements/plutonium.html">Plutonium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Plutonium." Chemicool Periodic Table. Chemicool.com. 21 Jul. 2017. Web. <https://www.chemicool.com/elements/plutonium.html>.