The chemical element lanthanum is classed as a lanthanide and rare earth metal. It was discovered in 1839 by Carl G. Mosander.

Data Zone
| Classification: | Lanthanum is a lanthanide and rare earth metal |
| Color: | silvery-white |
| Atomic weight: | 138.9055 |
| State: | solid |
| Melting point: | 1540 oC, 1813.2 K |
| Boiling point: | 2830 oC, 3103 K |
| Electrons: | 57 |
| Protons: | 57 |
| Neutrons in most abundant isotope: | 82 |
| Electron shells: | 2,8,18,18,9,2 |
| Electron configuration: | [Xe] 5d1 6s2 |
| Density @ 20oC: | 6.16 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 22.5 cm3/mol |
| Structure: | hcp: hexagonal close packed |
| Hardness: | 2.5 mohs |
| Specific heat capacity | 0.19 J g-1 K-1 |
| Heat of fusion | 6.20 kJ mol-1 |
| Heat of atomization | 414 kJ mol-1 |
| Heat of vaporization | 400 kJ mol-1 |
| 1st ionization energy | 538.1 kJ mol-1 |
| 2nd ionization energy | 1067 kJ mol-1 |
| 3rd ionization energy | 1850 kJ mol-1 |
| Electron affinity | 50 kJ mol-1 |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 3 |
| Max. common oxidation no. | 3 |
| Electronegativity (Pauling Scale) | 1.1 |
| Polarizability volume | 31.1 Å3 |
| Reaction with air | vigorous, w/ht ⇒ La2O3 |
| Reaction with 15 M HNO3 | mild, ⇒ La(NO3)3 |
| Reaction with 6 M HCl | mild, ⇒ H2, LaCl3 |
| Reaction with 6 M NaOH | none |
| Oxide(s) | La2O3 |
| Hydride(s) | LaH2, LaH3 |
| Chloride(s) | LaCl3 |
| Atomic radius | 195 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 117.2 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 13.4 W m-1 K-1 |
| Electrical conductivity | 1.9 x 106 S m-1 |
| Freezing/Melting point: | 1540 oC, 1813.2 K |

Lanthanum is used in many applications, such as studio lighting, laptop batteries, camera lenses and hybrid car batteries.
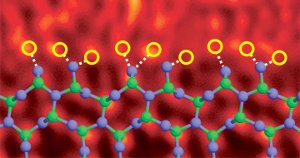
Silicon nitride grain boundary doped with lanthanum atoms. Image Ref. (6)
Discovery of Lanthanum
Lanthanum was discovered in 1839 by Carl G. Mosander in the mineral cerite in Stockholm, Sweden.
Ceria (cerium oxide) had already been discovered in 1803 by Swedish chemist Jacob Berzelius in the mineral cerite. Mosander, who had been one of Berzelius’s students, suspected that ceria was an impure oxide containing other rare earths. (1)
Mosander took finely powdered cerium nitrate and added cold dilute nitric acid. Some of the cerium nitrate powder dissolved in the acid indicating a new earth might be present. The new oxide was more basic than ceria (cerium oxide) and, unlike ceria, dissolved in the acid.
Mosander separated the solution from the precipitate using sodium oxalate and heat. He obtained a pale brick colored oxide of the new rare earth. (1)
The name lanthana for the new oxide was suggested by his friend Berzelius.
The name comes from the Greek word ‘lanthano’ meaning to be hidden.
Axel Erdmann discovered lanthanum independently in the same year as Mosander in a newly found Norwegian mineral. Erdmann called the new mineral mosandrite, in honor of Mosander. (2)
Mosander obtained impure metallic lanthanum from the chloride. (1)
Pure lanthanum metal was first produced in 1923 by electrolysis of the fused halides by H. Kremers and R. Stevens.
Mosander continued studying lanthana and in 1841 announced the discovery of another rare earth contained in it. He named it ‘didymium’ because it seemed to be ‘an inseparable twin brother of lanthanum.’ (2)
‘Didymium’ was later shown in separate discoveries to be a mixture of unknown rare earth elements. In 1879 Lecoq de Boisbaudran detected samarium in didymium and separated samarium from it. Carl Auer von Welsbach discovered in 1885 that the remaining ‘didymium’ was actually a mixture of two entirely new elements: neodymium and praseodymium.


Lanthanum is used in some pool products to reduce the level of phosphate nutrients that feed algae.

Clockwise from top center: Rare earth oxides of praseodymium, cerium, lanthanum, neodymium, samarium and gadolinium. Photo: LLNL
Appearance and Characteristics
Harmful effects:
Lanthanum and its compounds are considered to be moderately toxic.
Characteristics:
Lanthanum is a silvery-white soft metal, which can be cut with a knife.
It is ductile, malleable and exposed surfaces tarnish rapidly in air forming the oxide.
Lanthanum reacts with water to form lanthanum hydroxide plus hydrogen gas.
Lanthanum is chemically reactive and forms compounds with carbon, nitrogen, boron, selenium, silicon, phosphorus, sulfur, and with the halogens.
Lanthanum usually exists as a trivalent ion, La3+, in its compounds.
Uses of Lanthanum
Lanthanum is used in large quantities in nickel metal hydride (NiMH) rechargeable batteries for hybrid automobiles. The negative electrode (cathode) in NiMH batteries is a mixture of metal hydrides – one of which is typically lanthanum hydride. The active material at the cathode is hydrogen, which is stored in the metal hydride structure. The metal hydride can, depending on its composition, hold between 1% and 7% hydrogen by weight. (3) A Toyota Prius battery requires about 10 kg of lanthanum. (4)
Lanthanum is used as a petroleum cracking catalyst, catalyzing the splitting of long chain hydrocarbons into shorter chained species.
Lanthanum is used as an additive to make nodular cast iron and as an additive in steel.
Flame lighter flints use misch metal (a rare earth alloy) containing lanthanum to produce sparks by friction.
Lanthanum is used in hydrogen sponge alloys, which take up to 400 times their own volume of hydrogen gas.
Lanthanum is also used to make night vision goggles (infrared-absorbing glass).
High quality camera and telescope lenses contain lanthanum oxide (La2O3) making use of its high refractive index and low dispersion.
Lanthanum carbonate is used to reduce blood levels of phosphate in patients with kidney disease. (5)
Lanthanum compounds are also used in some pool products to reduce the level of phosphate nutrients that algae feed on.
Abundance and Isotopes
Abundance earth’s crust: 34 parts per million by weight, 5 parts per million by moles
Abundance solar system: 2 parts per billion by weight, 0.01 parts per billion by moles
Cost, pure: $800 per 100g
Cost, bulk: $ per 100g
Source: Lanthanum is not found free in nature. It is found mainly in the minerals monazite and bastnaesite. Commercially, it is recovered from monazite sand and bastnaesite using various complex extraction techniques. Pure lanthanum is obtained by the reduction of the fluoride with calcium metal.
Isotopes: Lanthanum has 31 isotopes whose half-lives are known, with mass numbers 119 to 150. Of these, one is stable, 139La. Naturally occurring lanthanum is a mixture of the two isotopes 138La and 139La with natural abundances of 0.09% and 99.91% respectively.

References
- Ferenc Szabadváry, Handbook of the Chemistry and Physics of the Rare Earths Vol. 11., Elsevier Science Publishers., 1998, p41-42
- Mary Elvira Weeks, Discovery of the Elements., Kessinger Publishing, 2003, p701-704
- John J.C. Kopera, Inside the Nickel Metal Hydride Battery., Cobasys 2004. pdf download.
- Robert Bryce, The Lanthanides, China, and High-Tech Autos., Toyota. pdf download.
- Lanthanum., Medline Plus.
- Photo: ORNL
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/lanthanum.html">Lanthanum</a>
or
<a href="https://www.chemicool.com/elements/lanthanum.html">Lanthanum Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Lanthanum." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web. <https://www.chemicool.com/elements/lanthanum.html>.
I have several options to store Lanthanum:
1) Mineral Oil
2) Argon sealed container
3) storing in a sealed glass container with oxygen absorbing bags.
4) wrapping in multiple polyethylene bags also with oxygen absorbing bags.
Please send me a comment on the last two options, as I don’t know how to inject Argon into a container, and the sample may become dirty with mineral oil.
Hi Mark,
I’ve no direct experience of working with Lanthanum (presumably we’re talking about the solid metal). To work with a reactive metal under an inert atmosphere I’d personally opt for a glove box. The link below isn’t a recommendation, it’s a result from a search for a suitable glovebox:
https://www.inerttechnology.com/gloveboxes/
Thanks Doug,
At the moment I am using the option 1), it is not ideal but it may stop oxidation. I am still searching for answers on 2) 3) and 4). Glove box is for working with it, not for storing it. I am going to purchase a glove box once I have the physical space in the laboratory.Thanks,