The chemical element carbon is classed as a nonmetal. It has been known since ancient times. Its discoverer and discovery date are unknown.

Data Zone
| Classification: | Carbon is a nonmetal |
| Color: | black (graphite), transparent (diamond) |
| Atomic weight: | 12.011 |
| State: | solid |
| Melting point: | 3550 oC, 3823 K |
| Note: At normal atmospheric pressure, carbon does not melt when heated, it sublimes. i.e. it undergoes a phase change directly from solid to gas. If the pressure is increased to 10 atmospheres carbon (graphite) is observed to melt at 3550 °C. | |
| Boiling point: | 3825 oC, 4098 K |
| The boiling point quoted is recorded when the graphite vapor pressure above subliming graphite reaches 1 atmosphere. | |
| Electrons: | 6 |
| Protons: | 6 |
| Neutrons in most abundant isotope: | 6 |
| Electron shells: | 2,4 |
| Electron configuration: | 1s2 2s2 2p2 |
| Density @ 20oC: | 2.267 g/cm3 (gr), 3.513 g/cm3 (di) |
Compounds, Radii, Conductivities
| Atomic volume: | 5.31 cm3/mol (gr), 3.42 cm3/mol (di) |
| Structure: | hexagonal layers (graphite), tetrahedral (diamond) |
| Hardness: | 0.5 mohs (graphite), 10.0 mohs (diamond) |
| Specific heat capacity | 0.71 J g-1 K-1 (graphite), 0.5091 J g-1 K-1 (diamond) |
| Heat of fusion | 117 kJ mol-1 (graphite) |
| Heat of atomization | 717 kJ mol-1 |
| Heat of vaporization | 710.9 kJ mol-1 |
| 1st ionization energy | 1086.5 kJ mol-1 |
| 2nd ionization energy | 2352.6 kJ mol-1 |
| 3rd ionization energy | 4620.5 kJ mol-1 |
| Electron affinity | 121.55 kJ mol-1 |
| Minimum oxidation number | -4 |
| Min. common oxidation no. | -4 |
| Maximum oxidation number | 4 |
| Max. common oxidation no. | 4 |
| Electronegativity (Pauling Scale) | 2.55 |
| Polarizability volume | 1.8 Å3 |
| Reaction with air | vigorous, ⇒ CO2 |
| Reaction with 15 M HNO3 | mild, w/ht ⇒ C6(CO2H)6 (mellitic/graphitic acid) |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | CO , CO2 |
| Hydride(s) | CH4 and many CxHy |
| Chloride(s) | CCl4 |
| Atomic radius | 70 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 25-470 W m-1 K-1 (graphite), 470 W m-1 K-1 (diamond) |
| Electrical conductivity | 0.07 x 106 S m-1 |
| Freezing/Melting point: | 3550 oC, 3823 K |
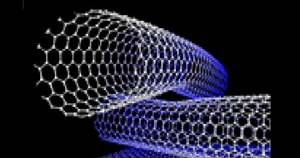
Models of carbon nanotube structure.
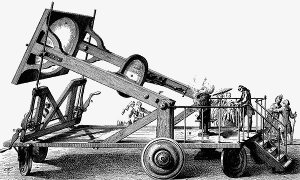
Lavoisier using a giant lens in combustion experiments
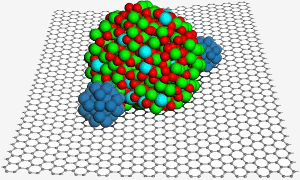
A graphene surface hosts an indium tin oxide nanoparticle, which helps secure two platinum nanoparticles (blue) for improved catalysis in a fuel cell. Image: PNL.
Discovery of Carbon
Carbon has been known since ancient times in the form of soot, charcoal, graphite and diamonds. Ancient cultures did not realize, of course, that these substances were different forms of the same element
French scientist Antoine Lavoisier named carbon and he carried out a variety of experiments to reveal its nature.
In 1772 he pooled resources with other chemists to buy a diamond, which they placed in a closed glass jar. They focused the sun’s rays on the diamond with a remarkable giant magnifying glass and saw the diamond burn and disappear.
Lavoisier noted the overall weight of the jar was unchanged and that when it burned, the diamond had combined with oxygen to form carbon dioxide. (1), (2) He concluded that diamond and charcoal were made of the same element – carbon.
In 1779, Swedish scientist Carl Scheele showed that graphite burned to form carbon dioxide and so must be another form of carbon.(3)
In 1796, English chemist Smithson Tennant established that diamond was pure carbon and not a compound of carbon; it burned to form only carbon dioxide.
Tennant also proved that when equal weights of charcoal and diamonds were burned, they produced the same amount of carbon dioxide. (4)
In 1855, English chemist Benjamin Brodie produced pure graphite from carbon, proving graphite was a form of carbon.(4)
Although it had been previously attempted without success, in 1955 American scientist Francis Bundy and coworkers at General Electric finally demonstrated that graphite could be transformed into diamond at high temperature and high pressure.(5)
In 1985, Robert Curl, Harry Kroto and Richard Smalley discovered fullerenes, a new form of carbon in which the atoms are arranged in soccer-ball shapes. The best known fullerene is buckminsterfullerene, also known as C60, consisting of 60 carbon atoms. A large family of fullerenes exists, starting at C20 and reaching up to C540. (6), (7)
The most recently discovered allotrope of carbon is graphene, which consists of a single layer of carbon atoms arranged in hexagons. If these layers were stacked upon one other, graphite would be the result. Graphene has a thickness of just one atom.
Graphene’s discovery was announced in 2004 by Kostya Novoselov and Andre Geim, who used adhesive tape to detach a single layer of atoms from graphite to produce the new allotrope.
Interesting Facts about Carbon
- About 20% of the weight of living organisms is carbon.
- More compounds are known which contain carbon than don’t.
- Carbon is the fourth most abundant element in the universe.
- Despite its high abundance, we owe Carbon’s existence to an unlikely set of circumstances
- Diamond is an excellent abrasive because it is the hardest common material and it also has the highest thermal conductivity. It can grind down any substance, while the heat generated by friction is swiftly conducted away.
- The carbon atoms in your body were all once part of the carbon dioxide fraction of the atmosphere.
- Graphene is the thinnest, strongest material ever known.
- Graphene is made of 2-dimensional atomic crystals, the first time such structures have ever been seen.
- The graphite in a typical mechanical pencil has a diameter of 0.7 mm. This is equal to 2 million layers of graphene.
- Car tires are black because they are about 30% carbon black, which is added to rubber to strengthen it. The carbon black also helps protect against UV damage to the tires.(8)
- Carbon is made within stars when they burn helium in nuclear fusion reactions. Carbon is part of the ‘ash’ formed by helium burning.
- Carbon undergoes nuclear fusion reactions in heavy stars to make neon, magnesium and oxygen.


Left: Combustion of coal (mainly amorphous carbon) in air. Right: Diamonds (crystalline carbon). We thought about taking a picture of combusting diamonds – they combust at about 800oC – but we couldn’t afford it!

Carbon based lifeforms, such as these, dominate our planet.

DNA. The famous double-helix molecule is made possible by carbon’s ability to form long molecular chains.
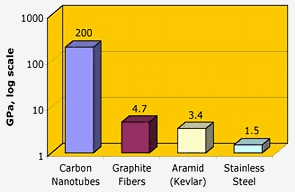
Nasa: Carbon nanotubes have outstanding tensile strength – two orders of magnitude higher than graphite fibers, kevlar or steel.
Carbon’s Periodic Table Neighborhood
| Group 13 |
Group 14 |
Group 15 |
|
| 2 | 5 B |
6 C |
7 N |
| 3 | 13 Al |
14 Si |
15 P |
| 4 | 31 Ga |
32 Ge |
33 As |
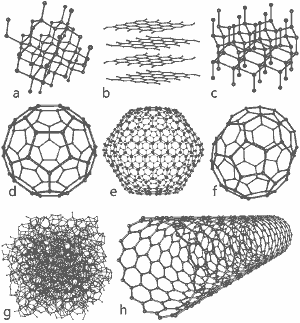
A wonderful image released by Michael Ströck under the GNU Free Documentation License: The structures of eight allotropes of carbon: a) Diamond b) Graphite c) Lonsdaleite d) C60 (Buckminsterfullerene) e) C540 Fullerene f) C70 Fullerene g) Amorphous carbon h) Single-walled carbon nanotube. Click here for larger image.
Appearance and Characteristics
Harmful effects:
Pure carbon has very low toxicity. Inhalation of large quantities of carbon black dust (soot/coal dust) can cause irritation and damage to the lungs.
Characteristics:
Carbon can exist with several different 3 dimensional structures in which its atoms are arranged differently (allotropes).
Three common crystalline allotropes are graphite, diamond, and (usually) fullerenes. Graphene has a 2D crystal structure.(Fullerenes can sometimes exist in amorphous form.)(9)
Carbon can also exist in an amorphous state. Many allotropes commonly described as amorphous, however, such as glassy carbon, soot, or carbon black usually have enough structure to not be truly amorphous. Although crystalline nanotubes have been observed, they are generally amorphous.(10)
The structures of eight allotropes are shown at the bottom of this page.
Interestingly, graphite is one of the softest substances and diamond was thought, until recently, to be the hardest naturally occurring substance.
An extremely rare allotrope of carbon, lonsdaleite, has been calculated, in pure form, to be 58% stronger than diamond. Lonsdaleite is a diamond-like carbon network which has graphite’s hexagonal structure. It is made when meteorites containing graphite hit another body, such as Earth. The high temperatures and pressures of the impact transform the graphite into lonsdaleite.
Carbon has the highest melting/sublimation point of all the elements and, in the form of diamond, has the highest thermal conductivity of any element.
Diamond’s high thermal conductivity is the origin of the slang term ‘ice.’ At typical room temperatures your body temperature is higher than the room’s – including any large diamonds you may just happen to have lying around. If you touch any of these diamonds, their high thermal conductivity carries heat away from your skin faster than any other material. Your brain interprets this rapid transfer of heat energy away from your skin as meaning you are touching something very cold – so diamonds at room temperature can feel like ice.
Uses of Carbon
Carbon (in the form of coal, which is mainly carbon) is used as a fuel.
Graphite is used for pencil tips, high temperature crucibles, dry cells, electrodes and as a lubricant.
Diamonds are used in jewelry and – because they are so hard – in industry for cutting, drilling, grinding, and polishing.
Carbon black is used as the black pigment in printing ink.
Carbon can form alloys with iron, of which the most common is carbon steel.
The 14C radioactive isotope is used in archaeological dating.
Carbon compounds are important in many areas of the chemical industry – carbon forms a vast number of compounds with hydrogen, oxygen, nitrogen and other elements.
Abundance and Isotopes
Abundance earth’s crust: 200 parts per million by weight, 344 parts per million by moles
Abundance solar system: 3000 parts per million by weight, 300 parts per million by moles
Cost, pure: $2.4 per 100g
Cost, bulk: $ per 100g
Source: Carbon can be obtained by burning organic compounds with insufficient oxygen. The four main allotropes of carbon are graphite, diamond, amorphous carbon and fullerenes.
Natural diamonds are found in kimberlite from ancient volcanoes.
Graphite can also be found in natural deposits.
Fullerenes were discovered as byproducts of molecular beam experiments in the 1980s.
Amorphous carbon is the main constituent of charcoal, soot (carbon black), and activated carbon.
Isotopes: 13 whose half-lives are known, with mass numbers 8 to 20. Naturally occurring carbon is a mixture of two isotopes and they are found in the percentages shown: 12C (99%) and 13C (1%).
Isotope 14C, with a half-life of 5730 years, is widely used to date carbonaceous materials such as wood, archeological specimens, etc. for ages up to about 40 000 years.

References
- Robert E. Krebs, The history and use of our earth’s chemical elements: a reference guide., (2006) p192. Greenwood Publishing Group
- Mary Elvira Weeks, The discovery of the elements. I. Elements known to the ancient world., J. Chem. Educ., 1932, 9 (1), p4
- Jessica Elzea Kogel, Industrial minerals & rocks: commodities, markets, and uses., (2006) p507. SME.
- Amanda S. Barnard, The diamond formula: diamond synthesis–a gemological perspective., (2000) p3. Butterworth-Heinemann
- Robert M. Hazen, The diamond makers., (1999) p145. Cambridge University Press.
- Jonathan W. Steed, Jerry L. Atwood, Supramolecular Chemistry., (2009) p423. Wiley.
- Nobel Prize for Chemistry, 1996
- What do we need to make a tire?
- Ming Gao and Hui Zhang, Preparation of an amorphous fullerene film., Physics Letters A Volume 213, Issues 3-4, 22 April 1996, Pages 203-206
- Ron Dagani, Nanotube Magic, Materials Research , April 16, 2001 Volume 79, Number 16 CENEAR 79 16 pp.6.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/carbon.html">Carbon</a>
or
<a href="https://www.chemicool.com/elements/carbon.html">Carbon Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Carbon." Chemicool Periodic Table. Chemicool.com. 25 July. 2014. Web. <https://www.chemicool.com/elements/carbon.html>.
Some very good material here, however, can anyone suggest sources for information below?
Is there a report or source that provides information on the electrical conductivity properties of commercially available activivated carbon or other forms of carbon?
Is there a report or source that provides information on the thermal conductivity properties of commercially available activivated carbon or other forms of carbon?
What is The Date Of Discovery Of Carbon
Do you mean discovery of carbon but in another form, (graphite, diamond etc, ) or when the first “discovery” that proved that those substances were different forms of carbon
are pencils pure carbon?
Hi nick, the part of the pencil that we write with is graphite, which is carbon.
Yes that´s right but most of the pencils that are sold contain a quite high amount of clay so it´s not “pure” graphite.
very interesting information.
What is the freezing point of carbon?
3550 degrees celcius