The chemical element nihonium is classed as an other metal. It was discovered in 2012 by Kosuke Morita’s RIKEN collaborative team.

Data Zone
| Classification: | Nihonium is an ‘other metal’ (presumed) |
| Atomic weight: | (286), no stable isotopes |
| State: | solid (presumed) |
| Melting point: | |
| Boiling point: | |
| Electrons: | 113 |
| Protons: | 113 |
| Neutrons in most abundant isotope: | 173 |
| Electron shells: | 2, 8, 18, 32, 32, 18, 3 |
| Electron configuration: | [Rn] 5f14 6d10 7s2 7p1 |
Reactions, Compounds, Radii, Conductivities
| Specific heat capacity | – |
| Heat of fusion | – |
| Heat of atomization | – |
| Heat of vaporization | – |
| 1st ionization energy | – |
| 2nd ionization energy | – |
| 3rd ionization energy | – |
| Electron affinity | – |
| Minimum oxidation number | – |
| Min. common oxidation no. | – |
| Maximum oxidation number | – |
| Max. common oxidation no. | – |
| Electronegativity (Pauling Scale) | – |
| Polarizability volume | – |
| Reaction with air | – |
| Reaction with 15 M HNO3 | – |
| Reaction with 6 M HCl | – |
| Reaction with 6 M NaOH | – |
| Oxide(s) | – |
| Hydride(s) | – |
| Chloride(s) | – |
| Atomic radius | – |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | – |
| Electrical conductivity | – |
| Freezing/Melting point: | – |

Nihonium was produced in a particle accelerator. Image: LLNL.
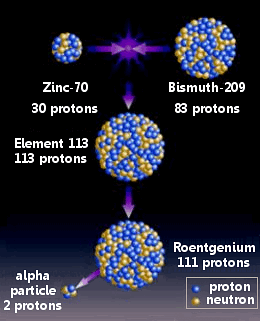
Nihonium was made by combining zinc-70 with bismuth-209 in a nuclear reation.
Discovery of Nihonium
Nihonium was discovered on August 12, 2012 by Kosuke Morita’s RIKEN collaborative team in Japan. It was the first chemical element ever discovered in Asia.
The discovery was formally accepted on December 30, 2015 by IUPAC and IUPAP, and a new superheavy element took its place in the seventh row of the periodic table.
The element is named after the place it was first synthesized; Nihon is one of two ways to say Japan in Japanese.
Nihonium was made using RIKEN’s Linear Accelerator Facility and the GARIS ion separator in Wako, Japan. The isotope produced was nihonium-278, which does not hang around for long: its half-life is less than a thousandth of a second.
Morita’s team had begun their work in September 2003. Zinc ions (70Zn) were formed into a beam in a particle accelerator and fired at a thin layer of bismuth (209Bi) in a cold fusion reaction.
Using this method the scientists believed they made a single atom of element 113 in July 2004 and again in April 2005. On each occasion the atom rapidly underwent four alpha decays: first to roentgenium-274, followed by meitnerium-270, bohrium-266, and dubnium-262.
Alpha decays :
278Nh ==> 274Rg ==> 270Mt ==> 266Bh ==> 262Db
These results were not sufficient to satisfy IUPAC and IUPAP. In 2011 their Joint Working Party refused to accept the discovery of element 113, stating that: “The work of the collaboration of Morita et al. is very promising but has not met the criteria for discovery owing to the paucity of events, the absence of firm connection(s) to known nuclides, and the inconsistencies noted above.”
The scientists in Japan then hit a dead end. Morita commented: “For over seven years we continued to search for data conclusively identifying element 113, but we just never saw another event. I was not prepared to give up, however, as I believed that one day, if we persevered, luck would fall upon us again.”
To help validate their discovery of nihonium, the team carried out a new experiment which would give them a better idea of the decay chain beyond 266Bh. A beam of sodium ions was collided with a curium target creating 266Bh which then decayed to 262Db.
On August 12, 2012 the scientists observed a third and conclusive decay event. Nihonium was created in the same way as before and underwent the same four alpha decays as previously. Additionally, 262Db continued to undergo alpha decays, yielding 258Lr followed by 254Md. As the chain had been fully characterized, this was taken as a clear demonstration that the source of the decay chain was indeed Nihonium, element 113.
In 2015 The IUPAC/IUPAP Joint Working Party (JWP) reviewed the work and stated that: “The RIKEN collaboration team in Japan have fulfilled the criteria for element Z=113 and will be invited to propose a permanent name and symbol.”
Appearance and Characteristics
Harmful effects:
Nihonium is harmful due to its radioactivity.
Characteristics:
Nihonium is a synthetic radioactive metal.
Uses of Nihonium
Nihonium is of research interest only.
Abundance and Isotopes
Abundance earth’s crust: nil
Abundance solar system: parts per trillion by weight, parts per trillion by moles
Cost, pure: $ per 100g
Cost, bulk: $ per 100g
Source: The element has been created using a cold fusion reaction between a bismuth-209 target and zinc-70 ions.
Isotopes: Nihonium has one isotope whose half-life is known, 278Nh.

References
- Experiment on the Synthesis of Element 113 in the Reaction, Journal of the Physical Society of Japan, Vol. 73, No. 10, October, 2004, pp. 2593–2596
- Observation of Second Decay Chain from 278113, Journal of the Physical Society of Japan Vol. 76, No. 4, April, 2007
- Robert Barber, Paul Karol, Hiromichi Nakahara, Emanuele Vardaci, and Erich Vogt, Discovery of the elements with atomic numbers greater than or equal to 113,. 2011, IUPAC. (pdf download)
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/ununtrium.html">Nihonium</a>
or
<a href="https://www.chemicool.com/elements/ununtrium.html">Nihonium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Nihonium." Chemicool Periodic Table. Chemicool.com. 11 Jun. 2016. Web. <https://www.chemicool.com/elements/ununtrium.html>.