The chemical element americium is classed as an an actinide metal. It was discovered in 1944 by Seaborg, James and Morgan.

Data Zone
| Classification: | Americium is an actinide metal |
| Color: | silvery-white |
| Atomic weight: | (243), no stable isotopes |
| State: | solid |
| Melting point: | 1176 oC, 1449 K |
| Boiling point: | 2607 oC, 2880 K |
| Electrons: | 95 |
| Protons: | 95 |
| Neutrons in most abundant isotope: | 148 |
| Electron shells: | 2,8,18,32,25,8,2 |
| Electron configuration: | [Rn] 5f7 7s2 |
| Density @ 20oC: | 13.67 g/cm3 |
Compounds, Radii, Conductivities
| Atomic volume: | 17.78 cm3/mol |
| Structure: | hcp: hexagonal close-packed |
| Hardness: | mohs |
| Specific heat capacity | 0.11 J g-1 K-1 |
| Heat of fusion | 14.40 kJ mol-1 |
| Heat of atomization | 266 kJ mol-1 |
| Heat of vaporization | 238.5 kJ mol-1 |
| 1st ionization energy | 578 kJ mol-1 |
| 2nd ionization energy | – |
| 3rd ionization energy | – |
| Electron affinity | – |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 6 |
| Max. common oxidation no. | 3 |
| Electronegativity (Pauling Scale) | 1.3 |
| Polarizability volume | 23.3 Å3 |
| Reaction with air | |
| Reaction with 15 M HNO3 | |
| Reaction with 6 M HCl | |
| Reaction with 6 M NaOH | |
| Oxide(s) | AmO, AmO2, Am2O3 |
| Hydride(s) | AmH2 AmH3 |
| Chloride(s) | AmCl2, AmCl3 |
| Atomic radius | 173 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 111.5 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 10 W m-1 K-1 |
| Electrical conductivity | 0.7 x 106 S m-1 |
| Freezing/Melting point: | 1176 oC, 1449 K |
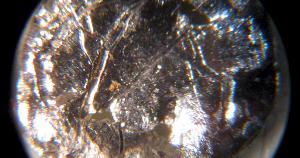
A small disc of americium-241 under the microscope. Image Ref.(3)
Discovery of Americium
Americium was the fourth synthetic transuranium element of the actinide series to be discovered.
Americium-241 was first identified in 1944 by Seaborg, James and Morgan at the metallurgical laboratory at the University of Chicago. It was produced by the beta-particle decay of plutonium-241, which had been produced in a nuclear reactor by neutron bombardment of plutonium-239.
The researchers at first referred to americium as “pandemonium” owing to the difficulties they encountered trying to isolate it from another new element with which it was very closely associated,
curium – or “delirium” as it was first called.
Americium was first isolated as a pure compound by Burris Cunningham in 1945, at the University of Chicago.
The element was named after America, because it is located below Europium (element 63) in the periodic table, which was named after Europe.
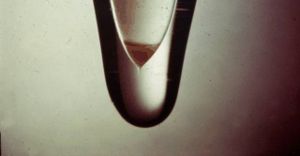
The triangle in the glass tube contains the world’s first sample of americium. (Photo credit: Berkeley Lab)
Appearance and Characteristics
Harmful effects:
Americium is harmful due to its radioactivity.
Characteristics:
Americium is a silvery-white highly radioactive metal that has a density similar to lead.
It tarnishes slowly in dry air at room temperature.
Isotope 241Am, the most common isotope, decays to 237Np, emitting alpha and gamma radiation(1).
Uses of Americium
Isotope 241Am is used (in the form of americium dioxide) in very small amounts in ‘ionization chamber’ smoke detectors. One gram of americium dioxide provides enough active material for more than three million household smoke detectors(2).
Americium is used as a portable source of gamma rays and alpha particles for use in medicine, science and industry.
It is also used as a target material in nuclear research to make even heavier elements.
Abundance and Isotopes
Abundance earth’s crust: nil
Abundance solar system: negligible
Cost, pure: $1500 per g
Cost, bulk: per 100g
Source: Americium is obtained as a by-product of plutonium processing.
Isotopes: Americium has 14 isotopes whose half-lives are known, with mass numbers 232 to 247. Americium has no naturally occurring isotopes. Its longest lived isotopes are 243Am, with a half-life of 7370 years, 241Am with a half-life of 432.2 years and 242Am with a half-life of 141 years.

References
- James D. Navratil, Wallace W. Schulz, Glenn T. Seaborg., The Most Useful Actinide Isotope: Americium-241., Journal of Chemical Education. 67.1 (1990): p15-16.
- Smoke Detectors and Americium., World Nuclear Association.
- Photo: Bionerd
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/americium.html">Americium</a>
or
<a href="https://www.chemicool.com/elements/americium.html">Americium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Americium." Chemicool Periodic Table. Chemicool.com. 15 Oct. 2012. Web. <https://www.chemicool.com/elements/americium.html>.
this was very helpful. could you put what the ionization energy has to be though?
Thanks for your comment Caledsa. Americium’s first ionization energy, the only one known, is 578 kJ/mol. The number is in the ‘Show More’ Data Zone near the top of the page.