The chemical element selenium is classed as a chalcogen and a nonmetal. It was discovered in 1818 by Jacob Berzelius.

Data Zone
| Classification: | Selenium is a chalcogen and a nonmetal |
| Color: | gray or red (crystalline), black or |
| red (amorphous) | |
| Atomic weight: | 78.96 |
| State: | solid |
| Melting point: | 220 oC, 493 K |
| Boiling point: | 685 oC, 958 K |
| Electrons: | 34 |
| Protons: | 34 |
| Neutrons in most abundant isotope: | 46 |
| Electron shells: | 2,8,18,6 |
| Electron configuration: | [Ar] 3d10 4s2 4p4 |
| Density @ 20oC: | 4.79 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 16.45 cm3/mol |
| Structure: | long, helical chains (crystalline hexagonal), Se8 |
| rings (crystalline monoclinic) | |
| Hardness: | 2.0 mohs |
| Specific heat capacity | 0.32 J g-1 K-1 |
| Heat of fusion | 6.694 kJ mol-1 |
| Heat of atomization | 227 kJ mol-1 |
| Heat of vaporization | 26.32 kJ mol-1 |
| 1st ionization energy | 940.9 kJ mol-1 |
| 2nd ionization energy | 2044.5 kJ mol-1 |
| 3rd ionization energy | 2973.7 kJ mol-1 |
| Electron affinity | 194.97 kJ mol-1 |
| Minimum oxidation number | -2 |
| Min. common oxidation no. | -2 |
| Maximum oxidation number | 6 |
| Max. common oxidation no. | 6 |
| Electronegativity (Pauling Scale) | 2.55 |
| Polarizability volume | 3.8 Å3 |
| Reaction with air | vigorous, w/ht ⇒ SeO2 |
| Reaction with 15 M HNO3 | mild , ⇒ H2SeO3, NOx |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | – |
| Oxide(s) | SeO2 |
| Hydride(s) | SeH2 |
| Chloride(s) | Se2Cl2, Se4Cl16 |
| Atomic radius | 119 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | 184 pm |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 0.52 W m-1 K-1 |
| Electrical conductivity | 8 x 106 S m-1 |
| Freezing/Melting point: | 220 oC, 493 K |

On average, each brazil nut contains 180 quadrillion selenium atoms. That’s 1.8 x 1017 Se atoms.
Discovery of Selenium
Selenium lies beneath sulfur in Group 16 of the periodic table. The chemical behavior and reactions of these elements are similar.
It is possible selenium was first observed in about the year 1300 by the alchemist Arnold of Villanova.
Villanova lived from about 1235 to about 1310 and was trained in medicine at the Sorbonne in Paris, becoming physician to Pope Clement V. In the book Rosarium Philosophorum he describes red sulfur or ‘sulfur rebeum’ which had been left behind in an oven after native sulfur had been vaporized. This may have been one of selenium’s red colored allotropes. (1), (2), (3)
There is no more to be said about selenium’s discovery until 500 years had passed.
In 1817 the eminent Swedish chemist Jacob Berzelius had his attention drawn to a red deposit left behind after sulfur had been burned in a sulfuric acid factory. (4)
The factory was actually part owned by Berzelius with his friend the chemist Johann Gahn. (5)
Writing about the deposit in September 1817, Berzelius informed his friend in London, Dr. Marcet, that the deposit contained the (already known) element tellurium.
In February 1818, however, he let Marcet know he had changed his mind, and told him of his discovery of a new element:
“…what Mr. Gahn and I took for tellurium is a new substance, endowed with interesting properties. This substance has the properties of a metal, combined with that of sulfur to such a degree that one would say it is a new kind of sulfur. The similarity to tellurium has given me occasion to name the new substance selenium.” (6)
To explain Berzelius’s name for the new element a little more: ‘Tellus’ means ‘earth goddess’ in Latin. Tellurium had been given its name in 1799 by the German chemist Martin Klaporth, who wrote: “No single element was yet named after the Earth. It needed to be done!” (7)
As a result of the new element’s similarity to tellurium, Berzelius named it selenium from the Greek word ‘Selene’ meaning ‘moon goddess.’


Allotropes of selenium. Top: amorphous black selenium; Middle: metallic gray selenium; Bottom: amorphous red selenium. Photo by Tomihahndorf.
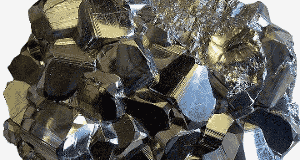
Pyrites, shown in the image, are mainly iron sulfide. The 1817 discovery of selenium was in sulfur extracted from pyrites. Photo by Aram Dulyan.
Appearance and Characteristics
Harmful effects:
Elemental selenium’s oral LD50 (the single dose needed to kill 50% of those exposed) is 6700 mg kg-1 in rats; this is similar to ethanol, which is 7000 mg kg-1. These levels are classed as non-toxic.
Selenium’s legal airborne permissible exposure limit (PEL) is 0.2 mg m-3 averaged over an 8-hour shift. The EPA describes selenium as not classifiable for human carcinogenicity. Selenium sulfide is a probable carcinogen.
Many of selenium’s compounds, such as selenates and selenites, are highly toxic.
Hydrogen selenide gas (SeH2) is selenium’s most acutely toxic compound.
Characteristics:
Selenium exists in several allotropic forms. The most stable form, crystalline hexagonal selenium, is metallic gray. Crystalline monoclinic selenium is a deep red color. Amorphous selenium is red in powder form and is black in vitreous form.
Gray crystalline ‘metallic’ selenium conducts electricity better in the light than in the dark (photoconductive) and it can convert light directly into electricity (photovoltaic).
In the same way as sulfur forms sulfides, sulfates, and sulfites, selenium combines with metals and oxygen to form selenides, (such as zinc selanide, ZnSe), selenates, (such as calcium selenate, CaSeO4), and selenites (such as silver selenite, Ag2SeO3).
Although hydrogen selenide gas (SeH2) is highly toxic, it’s unlikely you’ll hang around long enough to be poisoned; it has a disgusting smell. Oliver Sacks said, “Hydrogen selenide, I decided, was perhaps the worst smell in the world.” (8)
Uses of Selenium
Selenium is used in the glass industry to decolorize glass and to make red-colored glasses and enamels.
It is used as a catalyst in many chemical reactions.
Selenium is used in solar cells and photocells – in fact the first solar cell was made using selenium. It is also used as a photographic toner.
Selenium is used with bismuth in brasses and as an additive to stainless steel. When selenium is added to iron and copper based metals it improves their machinability.
Selenium sulfide is used in anti-dandruff shampoos.
Despite the toxicity of its compounds, selenium is also an essential trace element for humans and other animals. Without it, the enzyme glutathione peroxidase (GPX), which protects against oxidative damage in cells, could not function. Abnormally low selenium in the diet might increase the risk of cancer. Abnormally high levels of selenium compounds can lead to selenium poisoning. (9)
Plants do not appear to need selenium, but they do need sulfur. When selenium is present in soils, it is used by plants as if it were sulfur, introducing selenium into food chains. In soils with low sulfur content, some plants can have high levels of selenium compounds. Animals that eat these plants may suffer ill-health.
Selenium deficiency in animals can lead to slow growth and reproductive dysfunction.
Abundance and Isotopes
Abundance earth’s crust: 50 parts per billion by weight, 10 parts per billion by moles
Abundance solar system: parts per billion by weight, part per billion by moles
Cost, pure: $61 per 100g
Cost, bulk: $5.30 per 100g
Source: Selenium occasionally occurs free in nature, but more often occurs as selenides of iron, lead, silver, or copper. Commercially, selenium is obtained mainly from anode mud waste produced in the electrolytic refining of copper. Brazil nuts are the richest known dietary source of selenium.
Isotopes: Selenium has 24 isotopes whose half-lives are known, with mass numbers 67 to 91. Naturally occurring selenium is a mixture of six isotopes and they are found in the percentages shown: 74Se (0.9%), 76Se (9.4%), 77Se (7.6%), 78Se (23.8%), 80Se (49.7%) and 82Se (8.7%).

References
- Conor Reilly, Selenium in food and health, 1996, p2, Blackie Academic and Professional
- Francie Bauer, Selenium and Soils in the Western United States., 1997, Electronic Green Journal, UCLA Library, UC Los Angeles.
- Alastair Baxter, A Survey of the Occult., Edited by Julian Franklyn, 2005, p32, The Electric Book Company.
- Jöns J. Berzelius, Additional Observations on Lithion and Selenium, Annals of Philosophy, 1818, Volume 11, p373.
- Johan Erik Jorpes, Berzelius: his life and work.,1970 , p61, University of California Press.
- Mary Elvira Weeks, The discovery of the elements. VI. Tellurium and selenium, J. Chem. Educ., 1932, 9 (3), p474.
- Vivi Ringnes, Origin of the Names of Chemical Elements., J. Chem. Educ., 1989, 66 (9), p731.
- Oliver Sacks, Uncle Tungsten: Memories of a Chemical Boyhood, 2001, Knopf.
- Toxicological profile for selenium., 2003, p6, Agency for Toxic Substances and Disease Registry. (4.7 MB pdf download.)
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/selenium.html">Selenium</a>
or
<a href="https://www.chemicool.com/elements/selenium.html">Selenium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Selenium." Chemicool Periodic Table. Chemicool.com. 09 Oct. 2012. Web. <https://www.chemicool.com/elements/selenium.html>.