The chemical element gadolinium is classed as a rare earth metal. It was discovered in 1880 by Jean Charles Galissard de Marignac.

Data Zone
| Classification: | Gadolinium is a rare earth metal |
| Color: | silvery-white |
| Atomic weight: | 157.25 g/mol |
| State: | solid |
| Melting point: | 1314 oC, 1587 K |
| Boiling point: | 3260 oC, 3533 K |
| Electrons: | 64 |
| Protons: | 64 |
| Neutrons in most abundant isotope: | 94 |
| Electron shells: | 2,8,18,25,9,2 |
| Electron configuration: | [Xe] 4f7 5d1 6s2 |
| Density @ 20 oC: | 7.895 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 19.9 cm3/mol |
| Specific heat capacity | 0.23 J g-1 K-1 |
| Heat of fusion | 10.050 kJ mol-1 |
| Heat of atomization | 389 kJ mol-1 |
| Heat of vaporization | 311.71 kJ mol-1 |
| 1st ionization energy | 593.40 kJ mol-1 |
| 2nd ionization energy | 1170 kJ mol-1 |
| 3rd ionization energy | 1991 kJ mol-1 |
| Electron affinity | – |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 3 |
| Max. common oxidation no. | 3 |
| Electronegativity (Pauling Scale) | 1.20 |
| Polarizability volume | 23.5 Å3 |
| Reaction with air | mild, ⇒ Gd2O3 |
| Reaction with 15 M HNO3 | mild, ⇒ Gd(NO3)3 |
| Reaction with 6 M HCl | mild, ⇒ H2, GdCl3 |
| Reaction with 6 M NaOH | – |
| Oxide(s) | Gd2O3 |
| Hydride(s) | GdH2, GdH3 |
| Chloride(s) | GdCl3 |
| Atomic radius | 180 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 107.8 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 10.6 W m-1 K-1 |
| Electrical conductivity | 0.8 x 106 S m-1 |
| Freezing/Melting point: | 1314 oC, 1587 K |
Discovery of Gadolinium
In 1880, Swiss chemist Jean Charles Galissard de Marignac recorded previously unknown spectroscopic lines in an oxide preparation taken from the mineral samarskite. These were caused by the element we now know as gadolinium.
In 1886, French chemist Paul Émile Lecoq de Boisbaudran confirmed Marignac’s discovery. Boisbaudran suggested the name gadolinium for the new element after the 18th century chemist and mineralogist Johan Gadolin. The name was accepted by Marignac.
Earlier, in 1853, Marignac had discovered samarium by a similar method and he also discovered ytterbium in 1878.
Pure metallic gadolinium (99.3%) was first prepared by French chemist and engineer Felix Trombe in 1935. (1)
A few months later, Georges Urbain, Pierre Weiss and Felix Trombe discovered that gadolinium becomes ferromagnetic at about room temperature, the first pure element to show this property other than the three ‘classical’ metals iron, nickel, and cobalt.
They found that gadolinium becomes more ferromagnetic than iron but only at low temperatures. (1)


A distilled gadolinium cylinder.Image Ref.(4)
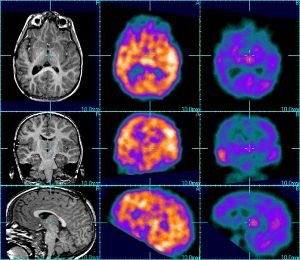
Gadolinium-153 is used in the calibration of single photon-emission computed tomography (SPECT) systems used for imaging in nuclear medicine. The SPECT images (middle and right) provide functional information about patient health, whereas the MRI image (left) provides only anatomical detail. Gadolinium-153 is produced in a nuclear reactor from europium or enriched gadolinium targets. Photo: Pacific Northwest National Lab

The left-most metal disc is cast dysprosium metal, resting on a sheet of sublimated dysprosium. The other metal disc is scandium and the metal cylinder is gadolinium. Photo: Ames Laboratory .

Clockwise from top center: Rare earth oxides of praseodymium, cerium, lanthanum, neodymium, samarium and gadolinium. Photo LLNL
Appearance and Characteristics
Harmful effects:
Harmful effects:
Gadolinium is considered to be moderately toxic.
Characteristics:
Gadolinium is a bright, soft, silvery-white metal that is both ductile and malleable.
It is one of the rare earth metals.
It does not react in dry air but will tarnish to a flaky white oxide in moist air that does not protect it from further oxidation.
The metal reacts slowly with water and is soluble in dilute acid. It produces colorless salts.
When present in compounds, gadolinium exists mostly in the trivalent state (Gd3+).
At room temperature the metal is paramagnetic, but it becomes ferromagnetic (strongly attracted by a magnet) when cooled. The Curie point of gadolinium is 17 oC. (2)
The 157Gd isotope has the highest thermal neutron capture cross-section of any known stable element.
Uses of Gadolinium
Gadolinium is used in alloys of iron and chromium to improve resistance to high temperatures and oxidation.
Gadolinium is used to make gadolinium yttrium garnets which have microwave applications.
Gadolinium compounds are used as green phosphors in color television picture tubes.
Because of its magnetic properties, gadolinium is also used in intravenous radiocontrast agents in magnetic resonance imaging (MRI).
Arc-melted alloys of gadolinium, silicon, and germanium demonstrate a strong magnetocaloric effect at room temperatures (where its temperature increases when it enters a magnetic field and decreases when it leaves the magnetic field) making it useful in the field of room temperature magnetic refrigeration. (3)
Abundance and Isotopes
Abundance earth’s crust: 5.2 parts per million by weight, 0.7 parts per million by moles
Abundance solar system: 2 parts per billion by weight, 10 parts per trillion by moles
Cost, pure: $450 per 100g
Cost, bulk: $12 per 100g
Source: Gadolinium is not found free in nature but is found in a number of minerals: mainly monazite and bastnaesite. Commercially, gadolinium is isolated by ion exchange and solvent extraction. The metal can be produced by the reduction of anhydrous gadolinium fluoride with calcium metal.
Isotopes: Gadolinium has 27 isotopes whose half-lives are known, with mass numbers 137 to 164. Naturally occurring gadolinium is a mixture of seven isotopes and they are found in the percentages shown: 152Gd (0.2%), 154Gd (2.2%), 155Gd (14.8%), 156Gd (20.5%), 157Gd (15.7%), 158Gd (24.8%) and 160Gd (21.9%). The most abundant isotope is 158Gd at 24.8%.

References
- T.S.S., The ferromagnetism of gadolinium., Current Science., August 1935. pdf
- T Lewowski and K Wozniak, Measurement of Curie temperature for gadolinium. pdf
- West Virginia University, Magnetic Refrigeration. pdf
- Image Ames Laboratory.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/gadolinium.html">Gadolinium</a>
or
<a href="https://www.chemicool.com/elements/gadolinium.html">Gadolinium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Gadolinium." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web. <https://www.chemicool.com/elements/gadolinium.html>.
can you use Gadolinium in a stress test/or has it ever been used as an intravenous radiocontrast agent for that purpose??