The chemical element neon is classed as a noble gas and a nonmetal. It was discovered in 1898 by William Ramsay and Morris Travers.

Data Zone
| Classification: | Neon is a noble gas and a nonmetal |
| Color: | colorless |
| Atomic weight: | 20.180 |
| State: | gas |
| Melting point: | -248.57 oC, 24.53 K |
| Boiling point: | -246.0 oC, 27.1 K |
| Electrons: | 10 |
| Protons: | 10 |
| Neutrons in most abundant isotope: | 10 |
| Electron shells: | 2,8 |
| Electron configuration: | 1s2 2s2 2p6 |
| Density @ 20oC: | 0.0009 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 16.7 cm3/mol |
| Structure: | fcc: face-centered cubic |
| Specific heat capacity | 0.904 J g-1 K-1 |
| Heat of fusion | 0.3317 kJ mol-1 |
| Heat of atomization | 0 kJ mol-1 |
| Heat of vaporization | 1.7326 kJ mol-1 |
| 1st ionization energy | 2080.6 kJ mol-1 |
| 2nd ionization energy | 3952.2 kJ mol-1 |
| 3rd ionization energy | 6121.9 kJ mol-1 |
| Electron affinity | – |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 0 |
| Max. common oxidation no. | 0 |
| Electronegativity (Pauling Scale) | – |
| Polarizability volume | 0.396 Å3 |
| Reaction with air | none |
| Reaction with 15 M HNO3 | none |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | none |
| Hydride(s) | none |
| Chloride(s) | none |
| Atomic radius | 38 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | – |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 0.05 W m-1 K-1 |
| Electrical conductivity | – |
| Freezing/Melting point: | -248.57 oC, 24.53 K |
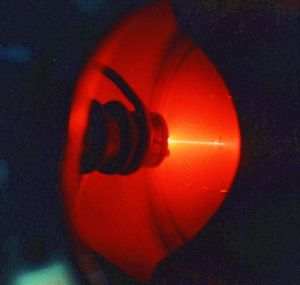
The glow which so excited Ramsay and Travers is from neon. The neon gas in this image is also excited – ionized and emitting light.

Neon gas spells ‘open’ with the help of a few thousand volts needed to ionize it.
Discovery of Neon
Neon was discovered in 1898 by William Ramsay and Morris Travers at University College London.
This was not the first time Ramsay had discovered a new element.
In 1894, he and Lord Rayleigh had discovered argon. Then, in 1895, Ramsay obtained the world’s first sample of helium. (Cleve and Langlet independently also obtained helium.)
Ramsay was aware an element must sit between helium and argon in the periodic table. But how could he find it?
Having found helium in a radioactive mineral, Ramsay thought it was possible he could find the new element in another such mineral. He and Travers spent some time working with a number of minerals, trying unsuccessfully to drive out some of the as yet undiscovered gas. (1)
Aware of the history of chemistry, Ramsay knew that sometimes one new element can hide another. For example, Berzelius discovered cerium in the mineral that came to be known as cerite. Some years later Mosander, one of Berzelius’s former students, who had continued to study cerite, discovered the new element lanthanum. Lanthanum had been present in the cerite all along, but Berzelius had not found it. Ramsay wondered about the possibility of finding small amounts of the elusive new element hiding in one of his earlier discoveries, argon.
Ramsay and Travers froze a sample of argon using liquid air. They then slowly evaporated the argon under reduced pressure and collected the first gas that came off.
To obtain the gas’s spectrum, Ramsay applied a high voltage to the gas in a vacuum tube and we may reasonably guess that his mouth fell open at what he saw.
Travers later commented, “the blaze of crimson light from the tube told its own story and was a sight to dwell upon and never forget… For the moment the actual spectrum of the gas did not matter in the least, for nothing in the world gave a glow such as we had seen.” (2)
This was the first time anyone had seen the glow of a neon light. Ramsay named the newly discovered element ‘neon’ which is Greek for ‘new.’
Interesting Facts about Neon
- 0.0018 percent of Earth’s atmosphere is neon.
- Although it is relatively rare on our planet, neon is the fifth most abundant element in the universe.
- If you could gather all the neon from the rooms in a typical new home in the United States, you would get 10 liters (2 gallons) of neon gas. (3),(4)
- Neon forms in stars with a mass of eight or more Earth suns. Near the end of their lives, these stars enter the carbon burning phase, also making oxygen, sodium and magnesium. (For oxygen production, stars need a mass of ‘just’ five of our suns.) (5),(6)
- Neon has no stable compounds.

Appearance and Characteristics
Harmful effects:
Neon is not known to be toxic.
Characteristics:
Neon is a light, very inert gas.
Colorless under normal conditions, it glows a reddish-orange in a vacuum discharge tube.
Neon forms no known stable compounds.
It has the smallest liquid range of any element (2.6 oC).
Uses of Neon
When a few thousand volts are applied to neon, it emits an orange/red light. It is therefore often used in brightly lit advertising signs. Georges Claude was the first person to make glass tubes of neon in 1910. He later bent the glass tubes to makes letters that glowed and produced the first neon advertising signs.
Neon is also used in high-voltage warning indicators, in Geiger counters and in television tubes.
Liquid neon is used as a cryogenic refrigerant.
Abundance and Isotopes
Abundance earth’s crust: 5 parts per billion by weight, 5 parts per billion by moles
Abundance solar system: 1,000 ppm by weight, 70 ppm by moles
Cost, pure: $33 per 100g
Cost, bulk: $ per 100g
Source: Neon is obtained commercially by fractional distillation of liquid air.
Isotopes: Neon has 14 isotopes whose half-lives are known, with mass numbers 16 to 29. Naturally occurring neon is a mixture of its three stable isotopes and they are found in the percentages shown: 20Ne (90.5%), 21Ne (0.7%) and 22Ne (9.2%).

References
- Mary Elvira Weeks, J. Chem. Educ., 1932, 9 (10), p 1751.
- Morris William Travers, The Discovery of the Rare Gases, 1928, Edward Arnold and Co.
- Room to swing a cat? Hardly BBC Report.
- Origin of the Earth’s Atmosphere.
- Post-Main Sequence Stars.
- William J. Kaufman III, Universe, 1987, W. H. Freeman and Company, New York, p434.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/neon.html">Neon</a>
or
<a href="https://www.chemicool.com/elements/neon.html">Neon Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Neon." Chemicool Periodic Table. Chemicool.com. 17 Oct. 2012. Web. <https://www.chemicool.com/elements/neon.html>.
I Liked This Website. The Only Thing I Couldnt Find Was Where Neon Can Be Currently Found&& How Its Made.. Thanks For The Help Tho. 🙂
Hi Carly Mariie13,
Neon is in the air all around us, but it’s only a small part of air: 54,900 liters of dry air contains 1 liter of neon.
Another way of saying this is that air contains 0.00182% neon by volume.
We get neon by fractional distillation of liquid air, which means we cool air until it’s a liquid. Then we can split it into the different gases that make it up by slowly heating it until the different gases boil off at different temperatures, depending on their individual boiling points.
That awkward moment when I’m using this website and I am a chemistry major……
The information on here is fabulous though!
i know right!. we learn things every day 🙂
I just started using this website. so far it is a cool website the element i am working in is number 10 neon.
Mighty cool presentation…….Thank you.
This site has a lot of useful information. I am in grade 8 and I was assigned to write information of the element of neon. This really helped and I couldn’t thank you enough
-Lauren