The chemical element uranium is classed as an actinide metal. It was discovered in 1789 by Martin Heinrich Klaproth.

Data Zone
| Classification: | Uranium is an actinide metal |
| Color: | silvery-white |
| Atomic weight: | 238.0289, no stable isotopes |
| State: | solid |
| Melting point: | 1135 oC, 1408 K |
| Boiling point: | 4130 oC, 4403 K |
| Electrons: | 92 |
| Protons: | 92 |
| Neutrons in most abundant isotope: | 146 |
| Electron shells: | 2,8,18,32,21,9,2 |
| Electron configuration: | [Rn] 5f3 6d1 7s2 |
| Density @ 20oC: | 18.9 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 12.59 cm3/mol |
| Structure: | orthorhombic |
| Hardness: | 6.0 mohs |
| Specific heat capacity | 0.12 J g-1 K-1 |
| Heat of fusion | 8.520 kJ mol-1 |
| Heat of atomization | 482 kJ mol-1 |
| Heat of vaporization | 422.58 kJ mol-1 |
| 1st ionization energy | 597.6 kJ mol-1 |
| 2nd ionization energy | 1420 kJ mol-1 |
| 3rd ionization energy | – |
| Electron affinity | – |
| Minimum oxidation number | 0 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 6 |
| Max. common oxidation no. | 6 |
| Electronegativity (Pauling Scale) | – |
| Polarizability volume | 27.4 Å3 |
| Reaction with air | mild, ⇒ U3O8 |
| Reaction with 15 M HNO3 | passivated |
| Reaction with 6 M HCl | mild, ⇒ H2 |
| Reaction with 6 M NaOH | none |
| Oxide(s) | UO, UO2, UO3 , U2O5, U3O8 |
| Hydride(s) | UH3 |
| Chloride(s) | UCl, UCl3, UCl4, UCl5, UCl6 |
| Atomic radius | 175 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | – |
| Ionic radius (3+ ion) | 116.5 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 27.5 W m-1 K-1 |
| Electrical conductivity | 3.6 x 10-6 S m-1 |
| Freezing/Melting point: | 1135 oC, 1408 K |
Discovery of Uranium
In ancient times uranium oxide was used to produce yellow colored ceramic glazes.
Uranium was formally discovered in 1789, in Berlin, Germany by Martin Heinrich Klaproth.
Klaproth was studying the mineral pitchblende, which was then believed to be a zinc/iron ore.
Klaproth dissolved pitchblende in nitric acid, then added potash to obtain a yellow precipitate. Adding excess potash dissolved the yellow precipitate. Such reactions were not characteristic of any known element and Klaproth concluded he had discovered a new element. (1)
He named it after the recently discovered planet Uranus.
In 1841, French chemist Eugene-Melchior Peligot isolated uranium metal by heating uranium tetrachloride with potassium.
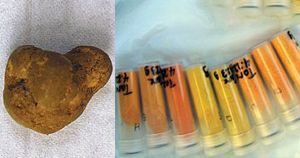
Below are photos of uranium ore (left) and uranium oxides in sample tubes. These oxides, known as yellowcake, are formed during the purification of uranium ores.


The former gas diffusion plant at Oak Ridge, conveying the scale of facilities needed to process fissile uranium.
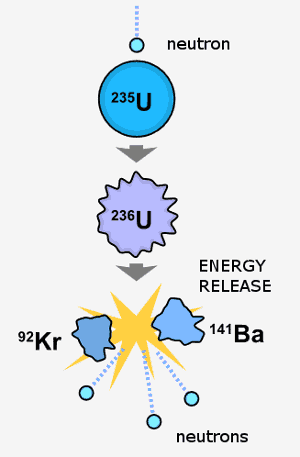
A neutron collides with a U-235 atom. U-236 forms briefly before it undergoes nuclear fission; this yields new atoms plus energy plus more neutrons. These newly released neutrons can cause more U-235 to split. If U-235 atoms are present in high enough concentrations, a nuclear chain reaction results. Image by Fastfission.
Appearance and Characteristics
Harmful effects:
Uranium is harmful both through its chemical toxicity and its radioactivity. Exposure to uranium increases your risk of getting a variety of cancers due to its radioactivity.
Characteristics:
Uranium is a dense, silvery-white, slightly paramagnetic, radioactive metal. It is also ductile and malleable. The metal tarnishes in air acquiring a dark layer of oxide. When finely powdered, uranium ignites spontaneously in air.
Uranium is a highly reactive metal and reacts with almost of all the nonmetallic elements and many of their compounds. It dissolves in acids, but it is insoluble in alkalis.
When present in compounds, uranium exists mostly in oxidation state IV and oxidation state VI.
All isotopes of uranium are radioactive, some more so than others. Its radioactivity – in particular its capacity to undergo thermonuclear chain reactions – has led to uranium’s use in energy generation, both for civilian and military purposes.
Uses of Uranium
Uranium is used as fuel for nuclear power plants. One kilogram of uranium-235 has the capacity to produce as much energy as 1,500,000 kilograms (1,500 tonnes) of coal.
Naturally occurring uranium is over 99% U-238 with only about 0.7% of the fissile U-235. Huge gas diffusion plants are used to produce enriched uranium, which has higher concentrations of U-235.
Uranium for use in nuclear power plants is enriched to a U-235 concentration of 2-3%.
In nuclear weapons, it is believed uranium is enriched to about 90% U-235, although lower concentrations would still yield a working bomb.
Depleted uranium is a byproduct of enriching uranium for nuclear purposes. It contains about 0.2% U-235 and is about half as radioactive as naturally occurring uranium.
Its lower radioactivity has allowed depleted uranium to be used in applications where uranium’s very high density is useful. (A tennis ball sized sphere of uranium would weigh about 5.7 pounds (2.6 kilograms).) It is used by the military as shielding to protect army tanks, and also in parts of bullets and missiles.
Use of depleted uranium in missiles is controversial because, on impact, uranium vapor and dust form and these are highly toxic.
U-238 can be converted into fissionable plutonium in breeder reactors.
Abundance and Isotopes
Abundance earth’s crust: 2.7 parts per million by weight, 0.25 parts per million by moles
Abundance solar system: 1 part per billion by weight, 4 parts per trillion by moles
Cost, pure: $ per 100g
Cost, bulk: $9 per 100g
Source: Uranium occurs naturally in several minerals such as uraninite (uranium oxide), carnotite and autunite. Canada is the world’s largest supplier of uranium, producing 20 to 30 percent of supplies. Commercially, uranium is produced through the reduction of uranium halides with alkali earth metals. Although most people think uranium is extraordinarily rare, it is in fact more abundant than familiar elements such as mercury and silver.
Isotopes: Uranium has 21 isotopes whose half-lives are known, with mass numbers 218 to 242. Natural uranium consists of three major isotopes: 234U, 235U, and 238U. All are radioactive. 238U is the most stable isotope, with a half-life of 4.51 x 109 years (almost the age of the Earth).

References
- Mary Elvira Weeks, Discovery of the Elements., (2003) p56. Kessinger Publishing Co.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/uranium.html">Uranium</a>
or
<a href="https://www.chemicool.com/elements/uranium.html">Uranium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Uranium." Chemicool Periodic Table. Chemicool.com. 24 Jul. 2015. Web. <https://www.chemicool.com/elements/uranium.html>.
what happens when a single atom of uranium is kept in a box and collided with a neutron ? Fission happens or not??
It would fissile but it would not be enough to create a chain reaction, because in order for fission to occur a large enough group of material needs to be present for the U235 to reach criticallity. (Chain reaction)
What happens to a uranium atom when it collides with uranium- 235?
nothing happens its simple science
There will be an elastic collision between the atoms. Kinetic energy and momentum will be conserved. The structure of the two atoms will be unaffected.
Is uranium considered a metal, metalloid, or nonmetal?
It’s a metal. It says so near the top of the page. 🙂
Is actual uranium used in nuclear reactors, or am I mixing something up?
Hi Darkness, the uranium in reactors is in the form of uranium dioxide. It doesn’t matter for the nuclear reaction whether the uranium is a pure metal or a compound. All that matters is getting the right number of uranium-235 atoms in each ml of the reactor.
The oxide is preferred to pure uranium because it has a much higher melting point (2865 °C) than the metal (1135 °C). Also, while uranium metal can burn, its oxide can’t. (Much like carbon can burn, but carbon dioxide is the product of burning, and it can’t burn more.)