The chemical element titanium is classed as a transition metal. It was discovered in 1791 by Reverend William Gregor.

Data Zone
| Classification: | Titanium is a transition metal |
| Color: | silvery-white |
| Atomic weight: | 47.87 |
| State: | solid |
| Melting point: | 1668 oC, 1941 K |
| Boiling point: | 3287 oC, 3560 K |
| Electrons: | 22 |
| Protons: | 22 |
| Neutrons in most abundant isotope: | 26 |
| Electron shells: | 2,8,10,2 |
| Electron configuration: | [Ar] 3d2 4s2 |
| Density @ 20oC: | 4.50 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 10.64 cm3/mol |
| Structure: | hcp: hexagonal close pkd |
| Hardness: | 6.0 mohs |
| Specific heat capacity | 0.52 J g-1 K-1 |
| Heat of fusion | 14.15 kJ mol-1 |
| Heat of atomization | 471 kJ mol-1 |
| Heat of vaporization | 425 kJ mol-1 |
| 1st ionization energy | 658 kJ mol-1 |
| 2nd ionization energy | 1310.3 kJ mol-1 |
| 3rd ionization energy | 2652.5 kJ mol-1 |
| Electron affinity | 7.6 kJ mol-1 |
| Minimum oxidation number | -1 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 4 |
| Max. common oxidation no. | 4 |
| Electronegativity (Pauling Scale) | 1.54 |
| Polarizability volume | 14.6 Å3 |
| Reaction with air | mild, w/ht ⇒ TiO2 |
| Reaction with 15 M HNO3 | passivated |
| Reaction with 6 M HCl | none |
| Reaction with 6 M NaOH | none |
| Oxide(s) | TiO, Ti2O3, TiO2 (titania) + more |
| Hydride(s) | TiH2 |
| Chloride(s) | TiCl2, TiCl3, TiCl4 |
| Atomic radius | 140 pm |
| Ionic radius (1+ ion) | 128 pm |
| Ionic radius (2+ ion) | 100 pm |
| Ionic radius (3+ ion) | 81 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 21.9 W m-1 K-1 |
| Electrical conductivity | 2.6 x 106 S m-1 |
| Freezing/Melting point: | 1668 oC, 1941 K |

The Guggenheim Museum, Bilbao, covered with titanium panels.

Ilmenite, the mineral in which William Gregor discovered titanium.
Discovery of Titanium
Titanium’s discovery was announced in 1791 by the amateur geologist Reverend William Gregor from Cornwall, England. (1), (2)
Gregor found a black, magnetic sand that looked like gunpowder in a stream in the parish of Mannacan in Cornwall, England. (We now call this sand ilmenite; it is a mixture consisting mainly of the oxides of iron and titanium.)
Gregor analyzed the sand, finding it was largely magnetite (Fe3O4) and the rather impure oxide of a new metal, which he described as ‘reddish brown calx.’
This calx turned yellow when dissolved in sulfuric acid and purple when reduced with iron, tin or zinc. Gregor concluded that he was dealing with a new metal, which he named manaccanite in honor of the parish of Mannacan.
Having discovered a new metal, Gregor returned to his pastoral duties.
Little more happens in our story until 1795, when the well-known German chemist Martin Klaproth experienced the thrill of discovering a new metallic element. Klaproth called the new metal titanium, after the Titans, the sons of the Earth goddess in Greek mythology.
Klaproth discovered titanium in the mineral rutile, from Boinik, Hungary. Just like Gregor’s calx, the rutile was a red color. In 1797 Klaproth read Gregor’s account from 1791 and realized that the red oxide in which he had found titanium and the red oxide in which Gregor had found manaccanite were in fact the same; titanium and maccanite were the same element and Gregor was the element’s true discoverer.
Gregor may have beaten Klaproth to the new metal, but scientists preferred Klaproth’s ‘titanium’ to Gregor’s ‘manaccanite.’
Obtaining a sample of pure titanium proved to be much harder than discovering it.
Many scientists tried, but it took 119 years from its discovery until 99.9% pure titanium was isolated in 1910 by metallurgist Matthew Hunter in Schenectady, New York, who heated titanium (IV) chloride with sodium to red-heat in a pressure cylinder. (2)
In 1936, the Kroll Process (heating titanium (IV) chloride with magnesium) made the commercial production of titanium possible. By 1948 worldwide production had reached just 3 tons a year.
By 1956, however, scientists and engineers had realized titanium’s properties were highly desirable and worldwide production had exploded to 25,000 tons a year. (3)
The 2011 forecast for worldwide production of titanium metal using the Kroll process was 223,000 metric tons. (4)

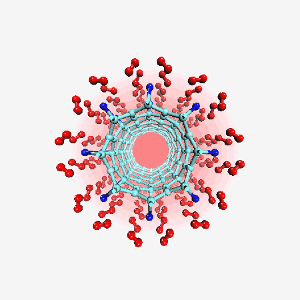
Computer generated image of titanium atoms (blue) bonded to a carbon nanotube in a hydrogen (red) fuel-cell. Molecules like this could improve the efficiency of fuel-cells for automotive use. Image: T. Yildirim/NIST
Appearance and Characteristics
Harmful effects:
Titanium metal is considered to be non-toxic. As metal shavings, or powder, it is a considerable fire hazard. Titanium chlorides are corrosive.
Characteristics:
Pure titanium is a light, silvery-white, hard, lustrous metal. It has excellent strength and corrosion resistance and also has a high strength to weight ratio.
Titanium’s corrosion rate is so low that after 4000 years in seawater, corrosion would only have penetrated the metal to the thickness of a thin sheet of paper. (3)
At high temperatures the metal burns in air and, unusually, titanium also burns in pure nitrogen.
Titanium is ductile and is malleable when heated.
It is insoluble in water, but soluble in concentrated acids.
Uses of Titanium
Titanium metal is used as an alloying agent with metals including aluminum, iron, molybdenum and manganese. Alloys of titanium are mainly used in aerospace, aircraft and engines where strong, lightweight, temperature-resistant materials are needed.
As a result of its resistance to seawater, (see above) titanium is used for hulls of ships, propeller shafts and other structures exposed to the sea.
Titanium is also used in joint replacement implants, such as the ball-and-socket hip joint.
About 95% of titanium production is in the forum of titanium dioxide (titania). This intensely white pigment, with a high refractive index and strong UV light absorption, is used in white paint, food coloring, toothpaste, plastics and sunscreen.
Titanium is used in several everyday products such as drill bits, bicycles, golf clubs, watches and laptop computers.
Abundance and Isotopes
Abundance earth’s crust: 0.56% by weight, 0.25% by moles
Abundance solar system: 4 parts per million by weight, 100 parts per billion by moles
Cost, pure: $661 per 100g
Cost, bulk: $ per 100g
Source: Titanium is the ninth most abundant metal in the Earth’s crust. Titanium is not found freely in nature but is found in minerals such as rutile (titanium oxide), ilmenite (iron titanium oxide) and sphene (titanite or calcium titanium silicate).
Commercially, the metal is isolated using the Kroll process which initially prepares titanium oxide from the mineral ilmenite. The oxide TiO2 is then converted to the chloride ( TiCl4) through carbochlorination. This is condensed and purified by fractional distillation and then reduced with molten magnesium in an argon atmosphere.
Isotopes: Titanium has 18 isotopes whose half-lives are known, with mass numbers 39 to 57. Naturally occurring titanium is a mixture of its five stable isotopes and they are found in the percentages shown: 46Ti (8.2%), 47Ti (7.4%), 48Ti (73.7%), 49Ti (5.4%) and 50Ti (5.2%). The most naturally abundant of these isotopes is 48Ti at 73.7%.

References
- William Gregor, Beobachtungen und Versuche über den Menakanite, einen in Cornwall gefundenen magnetischen Sand., in Lorenz Crell’s Chemische Annalen, 1791, p40.
- Mary Elvira Weeks, The discovery of the elements. XI. Some elements isolated with the aid of potassium and sodium: Zirconium, titanium, cerium, and thorium., J. Chem. Educ., 1932, p1231.
- Tom Margerison, The Future of Titanium., New Scientist Jun 12, 1958, p156.
- Research and development in titanium.
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/titanium.html">Titanium</a>
or
<a href="https://www.chemicool.com/elements/titanium.html">Titanium Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Titanium." Chemicool Periodic Table. Chemicool.com. 18 Oct. 2012. Web. <https://www.chemicool.com/elements/titanium.html>.
My teacher is making us do an adopt an element project, and the paper wants a melting (3,034°F 1,668°C) and boiling (5,948°F 3,287°C) point, but this element page doesn’t seem to show that, while others on this site do. other than that this site was super helpful! it was quite interesting to learn about titanium, and its uses. although as a side note some studies show that, titanium dioxide, is in fact toxic. However this has not been proven. Thanks for all your help!(yes, my name is spelled wrong on purpose)
Thanks for your comments Titainium. The melting and boiling points are in the data zone near the top of the page. 🙂
This is awesome. I found all the information I need for titanium. Thanks! 🙂
I am curious about titanium alloy as it is used as parts for pacemaker batteries and electrodes used for motor cortex stimulation. I recently had this metal implanted in my body and had a severe allergic reaction to both the battery and the brain implant leading to the entire device needing emergency removal November 1. 2017. What else is in the alloy?