The chemical element cobalt is classed as a transition metal. It was discovered in 1735 by George Brandt.

Data Zone
| Classification: | Cobalt is a transition metal |
| Color: | bluish-white |
| Atomic weight: | 58.9332 |
| State: | solid |
| Melting point: | 1495 oC, 1768 K |
| Boiling point: | 2930 oC, 3203 K |
| Electrons: | 27 |
| Protons: | 27 |
| Neutrons in most abundant isotope: | 32 |
| Electron shells: | 2,8,15,2 |
| Electron configuration: | [Ar] 3d7 4s2 |
| Density @ 20oC: | 8.90 g/cm3 |
Compounds, Radii, Conductivities
| Atomic volume: | 6.7 cm3/mol |
| Structure: | hcp: hexagonal close pkd |
| Hardness: | 5.0 mohs |
| Specific heat capacity | 0.42 J g-1 K-1 |
| Heat of fusion | 16.190 kJ mol-1 |
| Heat of atomization | 426 kJ mol-1 |
| Heat of vaporization | 373.3 kJ mol-1 |
| 1st ionization energy | 758.4 kJ mol-1 |
| 2nd ionization energy | 1646 kJ mol-1 |
| 3rd ionization energy | 3232.2 kJ mol-1 |
| Electron affinity | 63.8 kJ mol-1 |
| Minimum oxidation number | -1 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 5 |
| Max. common oxidation no. | 3 |
| Electronegativity (Pauling Scale) | 1.88 |
| Polarizability volume | 7.5 Å3 |
| Reaction with air | mild, w/ht ? Co3O4 |
| Reaction with 15 M HNO3 | vigorous, ? Co(NO3)2, NOx |
| Reaction with 6 M HCl | mild, ? H2, CoCl2 |
| Reaction with 6 M NaOH | – |
| Oxide(s) | CoO, Co3O4 |
| Hydride(s) | None |
| Chloride(s) | CoCl2 |
| Atomic radius | 135 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | 83.8 pm |
| Ionic radius (3+ ion) | 71.8 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 100 W m-1 K-1 |
| Electrical conductivity | 17.9 x 106 S m-1 |
| Freezing/Melting point: | 1495 oC, 1768 K |
Discovery of Cobalt
Since ancient times cobalt compounds have been used to produce blue glass and ceramics.
The element was first isolated by Swedish chemist George Brandt in 1735. He showed it was the presence of the element cobalt that caused the blue color in glass, not bismuth as previously thought.
In about 1741 he wrote, “As there are six kinds of metals, so I have also shown with reliable experiments… that there are also six kinds of half-metals: a new half-metal, namely cobalt regulus in addition to mercury, bismuth, zinc, and the reguluses of antimony and arsenic.”
The word cobalt is derived from the German ‘kobold’, meaning goblin or elf. The image of cobalt below is by Ben Mills.


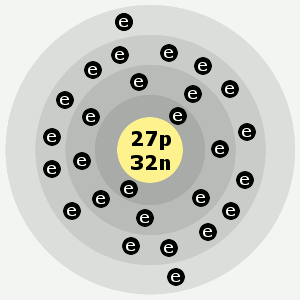
Cobalt’s electron shells
Appearance and Characteristics
Harmful effects:
Cobalt and its compounds are considered to be slightly toxic by skin contact and moderately toxic by ingestion.
Characteristics:
Cobalt is a bluish-white, lustrous, hard, brittle metal. It is ferromagnetic.
The metal is active chemically, forming many compounds. Cobalt stays magnetic to the highest temperature of all the magnetic elements (it has a Curie point of 1121oC).
Uses of Cobalt
Cobalt is used in alloys for aircraft engine parts and in alloys with corrosion/wear resistant uses.
Cobalt is widely used in batteries and in electroplating.
Cobalt salts are used to impart blue and green colors in glass and ceramics.
Radioactive 60Co is used in the treatment of cancer.
Cobalt is essential to many living creatures and is a component of vitamin B12.
Cobalt is also used in samarium-cobalt permanent magnets. These are used in guitar pickups and high speed motors.
Abundance and Isotopes
Abundance earth’s crust: 25 parts per million by weight, 8 parts per million by moles
Abundance solar system: 4 parts per million by weight, 0.7 parts per million by moles
Cost, pure: $21 per 100g
Cost, bulk: $4.40 per 100g
Source: Cobalt is not found as a free element in nature. It is found in mineral ores. The main ores of cobalt are cobaltite (CoAsS), erythrite (hydrated arsenate of cobalt), glaucodot (Co,Fe)AsS, and skutterudite (Co,Ni)As3. Cobalt is generally produced as a by-product of nickel and copper mining.
Isotopes: Cobalt has 22 isotopes whose half-lives are known, with mass numbers 50 to 72. Naturally occurring cobalt consists of its one stable isotope, 59Co.

References
- Edward Smedley, Hugh James Rose, Henry John Rose, Encyclopaedia Metropolitana; or Universal Dictionary of Knowledge., 1845, Volume 4, p693 William Clowes and Sons.
Cite this Page
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/cobalt.html">Cobalt</a>
or
<a href="https://www.chemicool.com/elements/cobalt.html">Cobalt Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Cobalt." Chemicool Periodic Table. Chemicool.com. 16 Oct. 2012. Web. <https://www.chemicool.com/elements/cobalt.html>.
This site is really helpful but I have a question: What does cobalt react with and what are the reactions?
Hi Kimmee, Cobalt can take part in a lot of reactions. We list some of these in the Data Zone near the top of this page. Scroll up to the Data Zone and click on “Show more: Heats, Energies, Oxidation, Reactions, Compounds, Radii, Conductivities”. You’ll find its reactions with air (oxygen), nitric acid, hydrochloric acid, and sodium hydroxide (no reaction).
Ok! Thank you so much! 🙂
What kinds of bonds does cobalt make with other elements? Like ionic or covalent?
Hi Hope, cobalt is a metal, so it forms ionic bonds.
This is a very helpful website for me and my fellow class mates, we are working on a assignment and it gave us a lot of information. Thank you:)