The chemical element iron is classed as a transition metal. It has been known since ancient times. Its discoverer and discovery date are unknown.

Data Zone
| Classification: | Iron is a transition metal |
| Color: | silvery-gray |
| Atomic weight: | 55.847 |
| State: | solid |
| Melting point: | 1535.1 oC, 1808.2 K |
| Boiling point: | 2750 oC, 3023 K |
| Electrons: | 26 |
| Protons: | 26 |
| Neutrons in most abundant isotope: | 30 |
| Electron shells: | 2,8,14,2 |
| Electron configuration: | [Ar] 3d6 4s2 |
| Density @ 20oC: | 7.87 g/cm3 |
Reactions, Compounds, Radii, Conductivities
| Atomic volume: | 7.1 cm3/mol |
| Structure: | bcc: body-centered cubic |
| Hardness: | 4.0 mohs |
| Specific heat capacity | 0.44 J g-1 K-1 |
| Heat of fusion | 13.80 kJ mol-1 |
| Heat of atomization | 415 kJ mol-1 |
| Heat of vaporization | 349.60 kJ mol-1 |
| 1st ionization energy | 759.3 kJ mol-1 |
| 2nd ionization energy | 1561.1 kJ mol-1 |
| 3rd ionization energy | 2957.3 kJ mol-1 |
| Electron affinity | 15.7 kJ mol-1 |
| Minimum oxidation number | -2 |
| Min. common oxidation no. | 0 |
| Maximum oxidation number | 6 |
| Max. common oxidation no. | 3 |
| Electronegativity (Pauling Scale) | 1.9 |
| Polarizability volume | 8.4 Å3 |
| Reaction with air | mild, ⇒ Fe3O4 |
| Reaction with 15 M HNO3 | passivated |
| Reaction with 6 M HCl | vigorous, ⇒ H2, FeCl2 |
| Reaction with 6 M NaOH | – |
| Oxide(s) | FeO, Fe2O3 (hematite), Fe3O4 (magnetite) |
| Hydride(s) | none |
| Chloride(s) | FeCl2, FeCl3 |
| Atomic radius | 140 pm |
| Ionic radius (1+ ion) | – |
| Ionic radius (2+ ion) | 77 pm |
| Ionic radius (3+ ion) | 63 pm |
| Ionic radius (1- ion) | – |
| Ionic radius (2- ion) | – |
| Ionic radius (3- ion) | – |
| Thermal conductivity | 80.4 W m-1 K-1 |
| Electrical conductivity | 11.2 x 106 S m-1 |
| Freezing/Melting point: | 1535.1 oC, 1808.2 K |
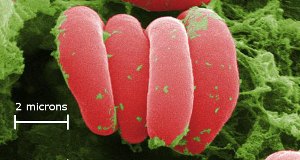
Red blood cells – the color comes from iron in hemoglobin. The cells are magnified x10,000. If you grew x10,000, you could place your feet in Seattle and touch Perth, Australia with your hands. Iron in hemoglobin carries oxygen around our bodies. Image Ref. (10)

Close up of an iron meteorite: Meteorites such as this were probably our ancestors’ first source of iron. This is a fragment of the Sikhote-Alin meteorite – approximately 93% iron, 6% nickel and 1% other elements. The meteorite surface has been melted into thumb-print shapes during its flight through our planet’s atmosphere. Photo by Carl Allen, NASA JSC Photo S94-43472.

Scrap iron and steel for recycling. How times have changed; iron was once worth eight times more than gold.
Discovery of Iron
Iron has been known since ancient times.
The first iron used by humans is likely to have come from meteorites.
Most objects that fall to earth from space are stony, but a small proportion, such as the one pictured, are ‘iron meteorites’ with iron contents of over 90 percent.
Iron corrodes easily, so iron artifacts from ancient times are much rarer that objects made of silver or gold. This makes it harder to trace the history of iron than the less reactive metals.
Artifacts made from meteorite iron have been found dating from about 5000 BC (and so are about 7000 years old) – for example iron beads in graves in Egypt. (1)
In Mesopotamia (Iraq) there is evidence people were smelting iron around 5000 BC.
Artifacts made of smelted iron have been found dating from about 3000 BC in Egypt and Mesopotamia. (1), (2), (3)
In those times, iron was a ceremonial metal; it was too expensive to be used in everyday life. Assyrian writings tell us that iron was eight times more valuable than gold. (1)
The iron age began about 1300-1200 BC when iron became cheap enough to replace bronze.
Adding carbon to iron to make steel was probably accidental at first – a coming together of molten iron and charcoal from the smelting fire. This probably happened about 1000 BC. (4)
Until this happened there were few technological reasons for the bronze age to give way to the iron age; the techniques of improving iron by adding carbon (to make steel) and coldworking were needed before iron would be wholly preferred to bronze. (5)
Iron was used commonly in Roman times. In the first century Pliny the Elder said, “It is by the aid of iron that we construct houses, cleave rocks, and perform so many other useful offices in life.” (6)
The origin of the chemical symbol Fe is from the Latin word ‘ferrum’ meaning iron. The word iron itself comes from ‘iren’ in Anglo-Saxon.
Interesting Facts about Iron
- One third of Earth’s mass is believed to be iron, most of which lies deep within the planet, in the core.
- Earth has enough iron to make three new planets, each with the same mass as Mars.
- Circulation of liquid iron deep in the earth is believed to create the electric currents that create our planet’s magnetic field.
- Iron is essential for human brain development. Iron deficiency in children leads to, among other problems, an impaired ability to learn. (7)
- In ancient times, people did not know how very abundant iron was on Earth. Their only source of metallic iron was meteorites. From Assyrian writings we learn that iron was eight times more valuable than gold. In addition to its rarity, iron may also have been very desirable because, coming from the sky, it was thought to be a gift from the gods: the ancient Egyptians called it ‘ba-ne-pe’, meaning ‘metal of heaven.’ The connection with heaven is reinforced by Pyramid Texts which translate, for example, to: ‘my bones are iron and my limbs are the imperishable stars.’ (8) (9)
- Iron was the first magnetic metal discovered. Lodestones were used by ancient navigators because they could be used as compasses, pointing to the magnetic north pole; this was described by the ancient Greek philosopher Thales of Miletus in 600 BC. Lodestones were made from magnetite, which is a naturally occurring oxide of iron. Magnetite’s formula is FeO.Fe2O3.
- Some animals have a sixth sense – the magnetic sense. Magnetite has been found in a wide range of animals, including honey bees, homing pigeons, and dolphins. These animals are sensitive to the earth’s magnetic field, helping their ability to navigate.
- The Hoba meteorite in Namibia is the largest naturally occurring piece of iron in the world, weighing in at over 60 tons. It’s made of 82 – 83% iron, 16 – 17 % nickel, about 1% cobalt, and very small traces of other elements. The Hoba meteorite is the largest single meteorite ever found.
- Iron is ferromagnetic. Ferromagnetism is the strongest type of magnetism. Other common ferromagnetic metals are nickel and cobalt.
- Very powerful magnets can be made using iron, nickel or cobalt in association with rare earth metals. NIB magnets (Neodymium – Iron – Boron) were invented in the early 1980s. They are an alloy in the proportions Nd2Fe14B. They are used in computers, cell phones, medical equipment, toys, motors, wind turbines and audio systems.



Appearance and Characteristics
Harmful effects:
Iron is considered to be non-toxic.
Characteristics:
Iron is a ductile, gray, relatively soft metal and is a moderately good conductor of heat and electricity.
It is attracted by magnets and can be readily magnetized.
The pure metal is chemically very reactive and rusts readily in moist air, forming red-brown oxides.
There are three allotropic forms of iron, known as alpha, gamma, and delta.
Alpha iron, also known as ferrite, is the stable form of iron at normal temperatures.
Uses of Iron
Iron is the cheapest and most important of all metals – important in the sense that iron is overwhelmingly the most commonly used metal, accounting for 95 percent of worldwide metal production.
Iron is used to manufacture steel and other alloys important in construction and manufacturing.
Iron is also vital in the functioning of living organisms, transporting oxygen in blood via the hemoglobin molecule.
Abundance and Isotopes
Abundance earth’s crust: 5.6 % weight, 2.1 % by moles
Abundance solar system: 1000 parts per million by weight, 30 parts per million by moles
Cost, pure: $7.2 per 100g
Cost, bulk: $0.02 per 100g
Source: Iron is not found free in nature but is found in iron ores such as hematite (Fe2O3), magnetite (Fe3O4) and taconite. Commercially, iron is produced in a furnace at temperatures of about 2000 oC by the reduction of hematite or magnetite with carbon.
Isotopes: Iron has 24 isotopes whose half-lives are known, with mass numbers 46 to 69. Naturally occurring iron is a mixture of four isotopes and they are found in the percentages shown: 54Fe (5.8%), 56Fe (91.8%), 57Fe (2.1%) and 58Fe (0.3%).

References
- Henry Maryon, Early Near Eastern Steel Swords., 65, 1961, American Journal of Archaeology p1.
- Michael D. Fenton, Mineral Commodity Profiles – Iron and Steel., 2005, U.S. Geological Survey.
- R. J. Forbes, Studies in Ancient Technology., IX, 1965, p247.
- Michael Woods, Mary B. Woods, Ancient Machines: From Wedges to Waterwheels., 2000, p30, Runestone Press.
- Vincent C. Pigott, The Archaeometallurgy of the Asian Old World, 1999, p28, UPenn Museum of Archaeology.
- Mary Elvira Weeks, Discovery of the Elements., 2003, p5, Kessinger Publishing.
- http://www.ncbi.nlm.nih.gov/pubmed/17101454.
- John G. Burke, Cosmic Debris: Meteorites in History., 1986, p229, University of California Press.
- Robert G. Bauval, Investigation on the origins of the benben stone. 14, 1989, Discussions in Egyptology.
- Image: CDC
Cite this Page
For online linking, please copy and paste one of the following:
<a href="https://www.chemicool.com/elements/iron.html">Iron</a>
or
<a href="https://www.chemicool.com/elements/iron.html">Iron Element Facts</a>
To cite this page in an academic document, please use the following MLA compliant citation:
"Iron." Chemicool Periodic Table. Chemicool.com. 06 Oct. 2012. Web. <https://www.chemicool.com/elements/iron.html>.
This Website Is Great To Find Information For Elements And More 😀
This website helped me on my science paper
ME2
this website really gave me great info for my science project! 🙂
This also really helped me for my Adopt an Element Project
This website is amazing it helped me with my science exam on elements, the periodic table and in depth facts and details.
Thanks Chemicool
This website will help me get an A+ on my project i now know that iron has a substance called hemoglobin which allows bloods cells to take in air and that is why are body needs iron.
this is really helpful