| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Period 1 |
1 H |
2 He |
||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
| 6 | 55 Cs |
56 Ba |
57-71 |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| 7 | 87 Fr |
88 Ra |
89-103 |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og |
| 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
||||
| 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
||||
Periodic Table Key
X Synthetic Elements |
X Liquids or melt at close to room temp. |
X Solids |
X Gases |
Alkali Metals |
Alkali Earth Metals |
Transition Metals |
Other Metals |
Metalloids |
Other Non Metals |
Halogens |
Noble Gases |
Lanthanides & Actinides |


Dmitri Mendeleev, Principles of Chemistry, Vol. 2, 1902, P. F. Collier, p17.

Henry Moseley, Philosophical Magazine, Vol. 26, 1913, p1030.

Carl Sagan, Cosmos, 1980, Random House, p223. Photo: NASA.
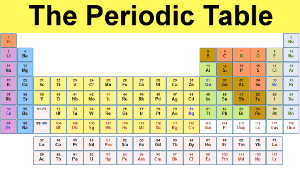
The Periodic Table
The periodic table we use today is based on the one devised and published by Dmitri Mendeleev in 1869.
Mendeleev found he could arrange the 65 elements then known in a grid or table so that each element had:
1. A higher atomic weight than the one on its left. For example, magnesium (atomic weight 24.3) is placed to the right of sodium (atomic weight 23.0):
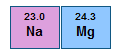
The True Basis of the Periodic Table
In 1913, chemistry and physics were topsy-turvy. Some big hitters - including Mendeleev - were talking seriously about elements lighter than hydrogen and elements between hydrogen and helium. Visualizing the atom was a free-for-all, and Mendeleev's justification for a periodic table based on atomic weights was falling apart at the seams.
This is the story of how Henry Moseley brought light to the darkness.
2. Similar chemical properties to other elements in the same column - in other words similar chemical reactions. Magnesium, for example, is placed in the alkali earths' column, with other elements whose reactions are similar:

Mendeleev realized that the table in front of him lay at the very heart of chemistry. And more than that, Mendeleev saw that his table was incomplete - there were spaces where elements should be, but no-one had discovered them.
Just as Adams and Le Verrier could be said to have discovered the planet Neptune on paper, Mendeleev could be said to have discovered germanium on paper. He called this new element eka-silicon, after observing a gap in the periodic table between silicon and tin:

Similarly, Mendeleev discovered gallium (eka-aluminum) and scandium (eka-boron) on paper, because he predicted their existence and their properties before their actual discoveries.
Although Mendeleev had made a crucial breakthrough, he made little further progress. With the benefit of hindsight, we know that Mendeleev's periodic table was underpinned by false reasoning. Mendeleev believed, incorrectly, that chemical properties were determined by atomic weight. Of course, this was perfectly reasonable when we consider scientific knowledge in 1869.
In 1869 the electron itself had not been discovered - that didn't happen for another 27 years.
In fact, it took all of 44 years for the correct explanation of the regular patterns in Mendeleev's periodic table to be found...
Read More... The Periodic Table continued