Chemistry Definition of Pauli's Exclusion Principle
The Pauli exclusion principle says that every electron must be in its own unique state. In other words, no electrons in an atom are permitted to have an identical set of quantum numbers.

The Pauli exclusion principle sits at the heart of chemistry, helping to explain the electron arrangements in atoms and molecules, and helping to rationalize patterns in the periodic table.
In chemistry the Pauli exclusion principle is applied solely to electrons, which we are about to discuss.
Wolfgang Pauli received the 1945 Nobel Prize in Physics for his discovery as it applied to electrons.
Later the Pauli exclusion principle was found to have a broader meaning, which we will mention at the end of this page.
Four Quantum Numbers
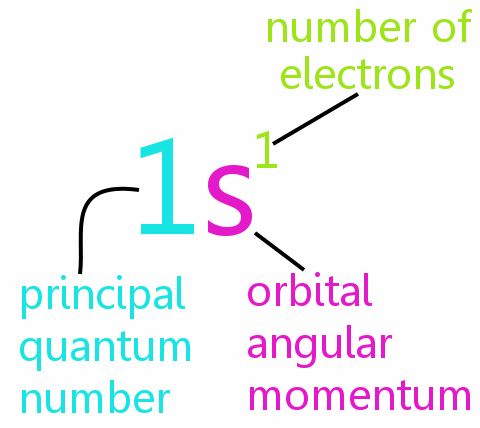
Every electron in an atom can be defined completely by four quantum numbers:
• n: the principal quantum number
• l: the orbital angular momentum quantum number
• ml: the magnetic quantum number
• ms: the spin quantum number
Example of the Pauli Exclusion Principle
Consider argon's electron configuration:
The exclusion principle asserts that every electron in an argon atom is in a unique state.
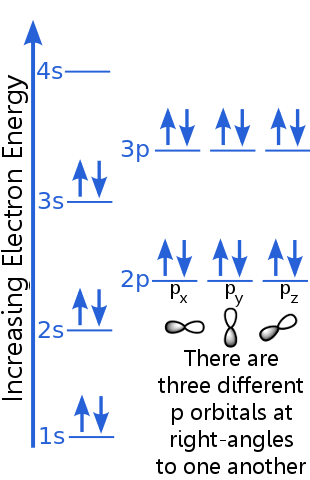
The 1s level can accommodate two electrons with identical n, l, and ml quantum numbers.
Argon's pair of electrons in the 1s orbital satisfy the exclusion principle because they have opposite spins, meaning they have different spin quantum numbers, ms. One spin is +½, the other is -½.
(Instead of saying +½ or -½ often the electrons are said to be spin-up ![]() or spin-down
or spin-down ![]() .)
.)
The 2s level electrons have a different principal quantum number to those in the 1s orbital. The pair of 2s electrons differ from each other because they have opposite spins.
The 2p level electrons have a different orbital angular momentum number from those in the s orbitals, hence the letter p rather than s. There are three p orbitals of equal energy, the px, py and pz. These orbitals are different from one another because they have different orientations in space. Each of the px, py and pz orbitals can accommodate a pair of electrons with opposite spins.
The 3s level rises to a higher principal quantum number; this orbital accommodates an electron pair with opposite spins.
The 3p level's description is similar to that for 2p, but the principal quantum number is higher: 3p lies at a higher energy than 2p.
General Definition of the Pauli Exclusion Principle
Electrons belong to a broad class of subatomic particles called fermions. Fermions have spin quantum numbers with half-integer values.
Quarks (up and down) and leptons (electrons, electron neutrinos, muons, muon neutrinos, taus, and tau neutrinos) are all fermions.
All fermions and particles derived from fermions, such as protons and neutrons, obey Fermi-Dirac statistics; this includes obeying the Pauli exclusion principle.
The Pauli exclusion principle says that no two identical fermions can simultaneously occupy the same quantum state.
The Pauli exclusion principle does not apply to bosons: these are particles that obey Bose-Einstein statistics; they all have integer values of spin. Photons, gluons, gravitons, and the W, Z and Higgs bosons are all bosons.
