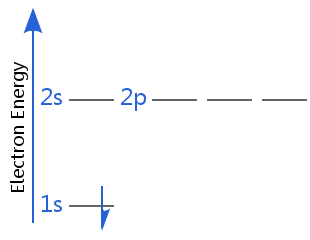The ground state of an atomic nucleus, atom, or molecule is its lowest energy state. Higher energy states are described as excited states.

The ground state applies to any quantized property of a particle. In chemistry,
• electron ground states
• vibrational ground states
• rotational ground states
of atoms and molecules are important, as are their excited states.
At room temperature, most molecules are in electron and vibrational ground states; higher temperatures are needed for molecules to enter excited states. However, most molecules are in an excited rotational state at room temperature, because less energy is needed for a molecule to enter a rotational excited state than an electron or vibrational excited state.
Electron Ground States
The diagrams below show electron energy levels for a hydrogen atom. In the first diagram, hydrogen is in its electron ground state.
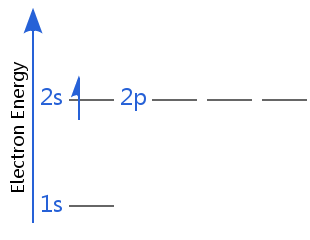
In the second diagram, hydrogen's electron is in a higher energy shell: hydrogen is no longer in its electron ground state; it is in an excited state. (Remember hydrogen, with one electron, has degenerate subshells, so 2s is degenerate with 2p - in all other atoms, electrons in the 2p subshell have higher energy than in the 2s.
If hydrogen's electron gained more energy it could enter higher sublevels such as 3s, 3p, 4s, 3d, etc. In all of these cases, it would be in an excited state.
Returning to the Ground State
Particles in excited states can return to the ground state by releasing energy. The energy is lost in the form of electromagnetic radiation.
- When electrons in atoms or molecules emit energy and fall into lower energy sublevels, including into the ground state, the energy takes the form of visible or ultraviolet light.
- Vibrational energy is lost by the emission of infrared light.
- Rotational energy is lost by the emission of microwave or far infrared radiation.
Degenerate Ground States
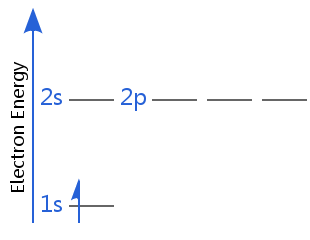
If there is more than one ground state, it means there is more than one lowest energy state.
For example, the diagrams below show two equal energy ground states for hydrogen. In one, the electron spin is +½; in the other it is -½.