For the transition elements in the fourth period, the 4d and 3d electron addition order requires an explanation that goes beyond the aufbau principle for lighter elements.
Here is a table of the electron configurations for elements 19-36, the fourth row of the periodic table. The transition elements scandium-zinc are shown in pink. For these elements, the electron energy order and filling order may at first seem unusual.
Period 4: Potassium - Krypton
| Element No. | Name | Electron Configuration |
|---|---|---|
| 19 | Potassium | [Ar] 4s1 |
| 20 | Calcium | [Ar] 4s2 |
| 21 | Scandium | [Ar] 3d1 4s2 |
| 22 | Titanium | [Ar] 3d2 4s2 |
| 23 | Vanadium | [Ar] 3d3 4s2 |
| 24 | Chromium | [Ar] 3d5 4s1 |
| 25 | Manganese | [Ar] 3d5 4s2 |
| 26 | Iron | [Ar] 3d6 4s2 |
| 27 | Cobalt | [Ar] 3d7 4s2 |
| 28 | Nickel | [Ar] 3d7 4s2 |
| 29 | Copper | [Ar] 3d10 4s1 |
| 30 | Zinc | [Ar] 3d10 4s2 |
| 31 | Gallium | [Ar] 3d10 4s2 4p1 |
| 32 | Germanium | [Ar] 3d10 4s2 4p2 |
| 33 | Arsenic | [Ar] 3d10 4s2 4p3 |
| 34 | Selenium | [Ar] 3d10 4s2 4p4 |
| 35 | Bromine | [Ar] 3d10 4s2 4p5 |
| 36 | Krypton | [Ar] 3d10 4s2 4p6 |
4s vs 3d Sublevels
At scandium, rather than a valence electron configuration of 4s2 3d1, we get 3d1 4s2.
This order is experimentally verified. For example, if scandium ionizes, it is the 4s electrons that are lost, not the 3d electron.
Electron Configurations of Scandium and its Ions
| Number of Electrons | Species | Electron Configuration |
|---|---|---|
| 21 | Sc | [Ar] 3d1 4s2 |
| 20 | Sc+ | [Ar] 3d1 4s1 |
| 19 | Sc2+ | [Ar] 3d1 4s0 |
| 18 | Sc3+ | [Ar] 3d0 4s0 |
Why do 4s and 3d Switch?
The energy levels of an atom's electrons are influenced by a number of parameters, such as the nuclear charge and electron-electron repulsions.
When there are fewer than 20 protons in the nucleus, the 4s sublevel is lower than the 3d sublevel.
However, for 20 protons or more, 3d drops below 4s.
Sublevels to Calcium
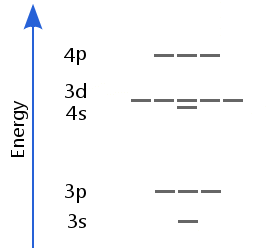
Transition Element Sublevels
Scandium has three valence electrons. The aufbau principle tells us they should add to the lowest available energy sublevel. Therefore, the first electron goes into 3d.
However, it turns out to be more favorable for the second to enter 4s. This is because when there are 20 protons or more, the 3d orbitals are more compact than 4s. The higher repulsion energy of a second electron entering 3d makes it unfavorable compared with the second electron entering 4s. Likewise, the third electron goes into 4s because it is the lowest cost energy option.
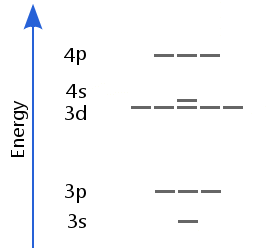
The Chromium Anomaly
Chromium has 24 protons and electrons. From the above, we might expect its electron configuration to be [Ar] 3d4 4s2.
In fact, it is [Ar] 3d5 4s1.
To explain this, again, we must bear in mind that the energy levels of the 3d and 4s are very similar. In chromium's case, the second option has lower energy because it the higher number of parallel spins produces the lowest energy configuration. This is in accordance with the principles of Hund's rule.
