The aufbau principle says that the arrangement of electrons in an atom - the electron configuration - is best understood if it is built from the ground up.
'Aufbauprinzip' is a German noun; it means 'construction principle.'
When writing down an atom's electron configuration, we begin at the lowest energy level and add electrons to higher energy sublevels until the required number of electrons are present.
We do so in accordance with the Pauli Exclusion Principle and Hund's Rule.
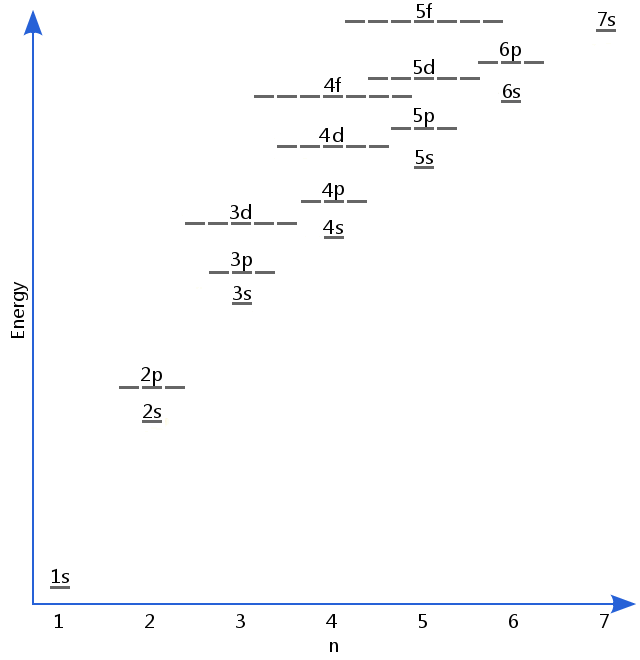
The diagram below shows as many electron energy levels as we need for most purposes in general chemistry, although higher levels also exist. Energy levels are filled from the bottom up.
Aufbau and the Elements - Period 1
Element 1 - Hydrogen
The simplest element, hydrogen, has one electron.
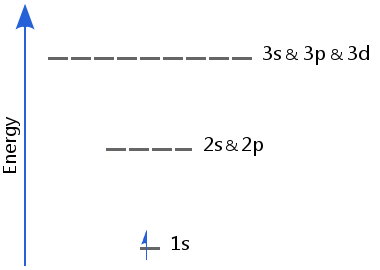
The aufbau principle places hydrogen's electron in the lowest available energy level, the 1s.
This is hydrogen's ground state. It is written 1s1.
Hydrogen is unique among the elements - it is the only one whose atoms have just one electron. As a result of this, hydrogen has unique energy levels.
The lack of electron-electron repulsion in hydrogen means its sublevels are of equal energy: in hydrogen 2s and 2p are of equal energy; likewise, 3s, 3p, and 3d are of equal energy, etc.
Element 2 - Helium
Helium's two electrons fill the 1s orbital, giving a ground state electron configuration of 1s2.
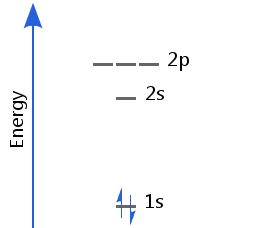
One electron is spin-up and the is other spin-down in accordance with the Pauli exclusion principle. This is called spin pairing.
The quantum numbers of one of helium's electrons are:
And the other electron's quantum numbers are:

Aufbau and the Elements - Period 2
Element 3 - Lithium
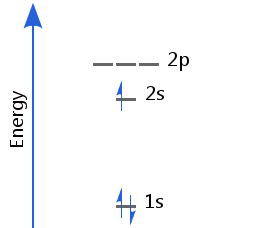
Lithium's first two electrons fill the 1s orbital; the third goes into the 2s, which is the next lowest available level. Lithium's electron configuration is 1s2 2s1.
Element 4 - Beryllium
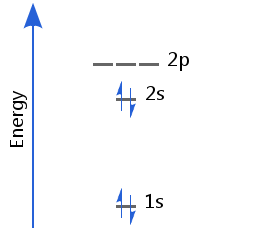
Beryllium's first two electrons fill the 1s orbital; the second two fill the 2s, with opposite spins. Beryllium's electron configuration is 1s2 2s2.
Element 5 - Boron
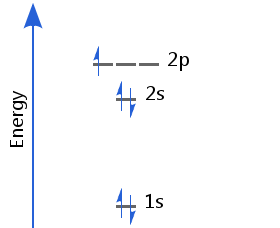
With the 1s and 2s sublevels full, boron's fifth electron occupies one of the p orbitals. Boron's electron configuration is 1s2 2s2 2p1.
Element 6 - Carbon
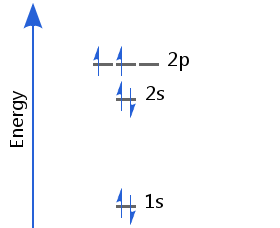
The sixth electron enters a different p orbital from the first. In accordance with Hund's rule, the two electrons in p orbitals have parallel spins. Carbon's electron configuration is 1s2 2s2 2p2.
Element 7 - Nitrogen
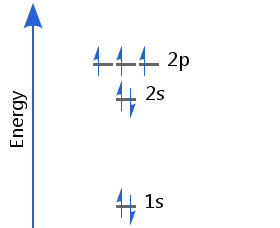
The seventh electron enters the p orbitals. In accordance with Hund's rule, the three electrons in p orbitals have parallel spins. Nitrogen's electron configuration is 1s2 2s2 2p3.
Element 8 - Oxygen
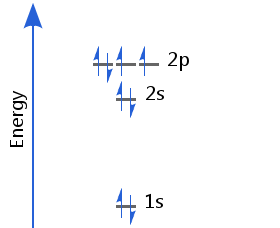
The eighth electron must now enter a p orbital already occupied by another. It enters with a pair spin orientation. Oxygen's electron configuration is 1s2 2s2 2p4.
Element 9 - Fluorine
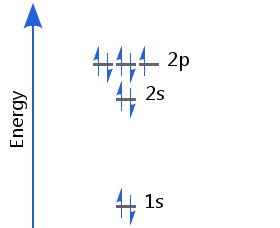
The ninth electron enters a p orbital. This electron's spin pairs with that of the electron already there. Fluorine's electron configuration is 1s2 2s2 2p5.
Element 10 - Neon
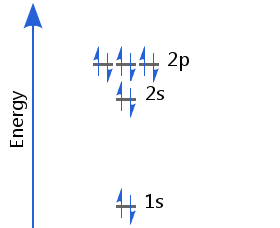
The tenth electron completes the occupation of the 2p sublevel. Neon's electron configuration is 1s2 2s2 2p6.
Neon's second energy shell (with principal quantum number, n = 2) is full. This is a particularly stable configuration, resulting in neon's lack of chemical reactivity.
Aufbau and the Elements - Period 3
Elements 11 - 18
For the next eight elements, the 3s and 3p sublevels fill until the next noble gas, argon is reached. The procedure is the same as the filling of the 2s and 2p sublevels.
Sodium - Argon
| Element No. | Name | Full Configuration | Shorthand Configuration |
|---|---|---|---|
| 11 | Sodium | 1s2 2s2 2p6 3s1 | [Ne] 3s1 |
| 12 | Magnesium | 1s2 2s2 2p6 3s2 | [Ne] 3s2 |
| 13 | Aluminum | 1s2 2s2 2p6 3s2 3p1 | [Ne] 3s2 3p1 |
| 14 | Silicon | 1s2 2s2 2p6 3s2 3p2 | [Ne] 3s2 3p2 |
| 15 | Phosphorus | 1s2 2s2 2p6 3s2 3p3 | [Ne] 3s2 3p3 |
| 16 | Sulfur | 1s2 2s2 2p6 3s2 3p4 | [Ne] 3s2 3p4 |
| 17 | Chlorine | 1s2 2s2 2p6 3s2 3p5 | [Ne] 3s2 3p5 |
| 18 | Argon | 1s2 2s2 2p6 3s2 3p6 | [Ne] 3s2 3p6 |
The 3d energy sublevel is considerably higher than the 3p. Therefore 3s and 3p in effect form a closed shell with the result that argon has very low reactivity.
Aufbau and the Elements - Period 4
The order that electrons add to an atom's sublevels is often described using the Madelung Rule, summarized in the diagram below.
Unfortunately, beyond the first 20 elements, the Madelung Rule is unreliable.
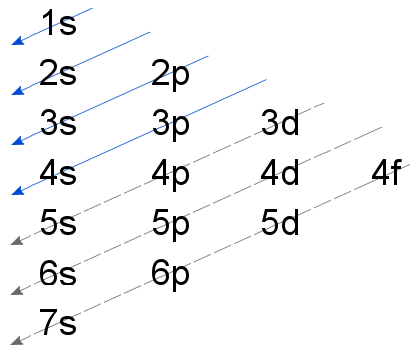
For the elements in the fourth row of the periodic table, the 4d and 3d sublevel order requires some explanation. This is provided on a seperate page: The Aufbau Principle and Transition Elements.
