Electrolytic cells convert electrical energy into chemical potential energy. The process is known as electrolysis. The purpose of this is usually to convert reactants into more useful products.
Electrolytic cells are one of two major categories of electrochemical cell.
The other category, voltaic cells, convert chemical potential energy to electrical energy. Battery-powered devices get their electrical energy from one or more voltaic cells.
Thermodynamics
Electrolytic cells use electrical energy to drive non-spontaneous chemical reactions - i.e. those reactions for which ΔG ≥ 0.
Examples of Electrolytic Cells
1. Manufacturing Sodium
Electrolysis is used to manufacture sodium metal from sodium chloride.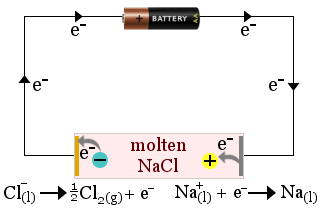
2. Recharging Batteries
Electrolysis is used to recharge rechargeable batteries: rechargeable batteries operate as voltaic cells when they are powering devices and as electrolytic cells during recharge.
For example, the Edison battery is a simple, rechargeable cell invented by Thomas Edison. It consists of two metal electrodes, one made of iron, the other of nickel. During initial charging, a coating of nickel oxide forms on the nickel electrode.
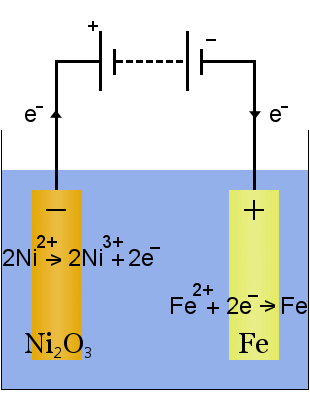
The electrolyte (the ionic liquid between the electrodes) is aqueous potassium hydroxide.
When it is discharging, the Edison cell operates as a voltaic cell. When it is being charged, the cell operates as an electrolytic cell.
The chemical equations for the reactions at the electrodes are:
During discharge, when the cell is delivering electrical energy, the reactions above proceed from left to right.
During charging, when the cell is operating electrolytically, converting electrical energy to chemical potential energy, the reactions above proceed from right to left.
3. Electrolysis of Water
Electrolysis of water produces the ultimate in clean fuels; one of chemistry's Holy Grails is to split water using sunlight.
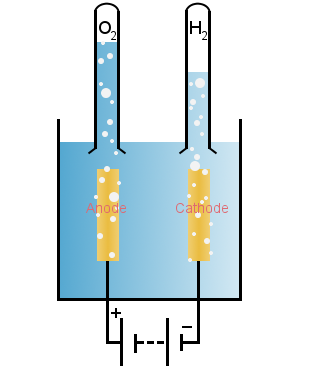
Direct current can be used to split water, as shown in the diagram
At the cathode, hydrogen ions gain electrons:
At the anode, water loses electrons, forming oxygen and hydrogen ions:
