At its simplest, an electrochemical cell consists of two electron conductors separated by an ionic conductor and linked by an electron conductor.
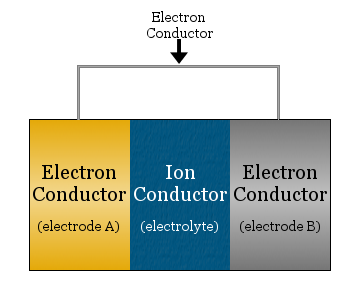
- the ionic conductor is called the electrolyte
- the electron conductors separated by the electrolyte are called electrodes
- the electron conductor used to link the electrodes is often a metal wire, such as copper wiring
Two Types of Cell
There are two fundamental types of electrochemical cell: galvanic and electrolytic.
Examples
- A battery powering something is an example of a galvanic cell.
- Rechargeable batteries are examples of both types of cell: they operate as galvanic cells when they are powering a device and as electrolytic cells when they are being recharged.
- Examples of electrolytic cells also include those used to split water into hydrogen and oxygen, and those that convert aluminum ore to aluminum metal.
A Source of Thermodynamic Data
In addition to practical uses, such as those described above, electrochemical cells provide an excellent way of gathering thermodynamic data. For example, they allow standard redox potentials to be determined, from which standard enthalpy, standard entropy and standard Gibbs free energy data for reactions can be obtained conveniently.
Separating Reactions
Electrochemical cells can:
- supply electricity, or
- convert metal ores to the metal, or
- provide thermodynamic data,
because the redox reactions take place separately.
Example: The Daniell Cell
In electrochemical cells, reactions take place at separate electrodes - and indeed the electrodes themselves may be placed in different vessels. Consider one of the early galvanic cells, the Daniell cell.
The Daniell cell's source of energy is the spontaneous reaction of zinc metal with copper sulfate to produce zinc sulfate and copper metal.
ΔG° = -212.6 kJ mol-1
When the materials are brought together:
- in direct contact in one vessel, chemical potential energy is converted into thermal energy and the reaction vessel gets warmer
- in an electrochemical cell, chemical potential energy is converted into electrical energy
The Daniell Cell
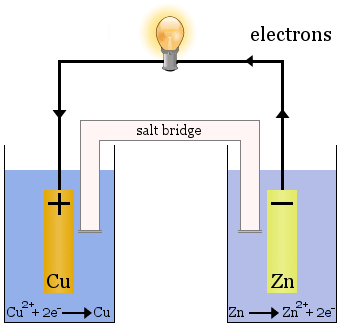
The Daniell cell is made up of two half-cells: copper ions are reduced in one and zinc is oxidized in the other.
When the cell operates, Cu2+ is removed from one cell and Zn2+ is produced in the other cell. The copper electrode begins to grow as it is plated with freshly deposited copper metal, and the zinc electrode begins to diminish due to the loss of zinc as ions into solution.
The salt bridge in the case above is glass tubing filled with saturated KNO3 solution. It has frits on the ends that prevent mixing of the solutions in each of the half-cells, but it allows ions to pass through to complete the electric circuit and keep each half-cell electrically neutral.
The driving force is the same ΔG° = -212.6 kJ mol-1 that is obtained when simply mixing the reactants in one vessel; by separating the two half-reactions, the electrons must travel through the wire and we can use the electrical energy.
