A voltaic cell, often known as a galvanic cell, provides electrical energy. The source of this energy is a spontaneous chemical reaction, more specifically a spontaneous redox reaction.
For example, all batteries are made of one or more voltaic cells; batteries go flat when most or all of their reactants have been converted to products, transforming their chemical potential energy to electrical energy.
Voltaic Cells - The Basic Process
Chemical Potential Energy → Electrical Energy
The voltaic cell is one of two basic types of electrochemical cell. The other type is the electrolytic cell; in an electrolytic cell, electrical energy is used to drive a non-spontaneous chemical reaction. For example, water can be split into hydrogen and oxygen in an electrolytic cell. Also, when a rechargeable battery is recharged, it operates as an electrolytic cell.
Basic Voltaic
Cell Schematic
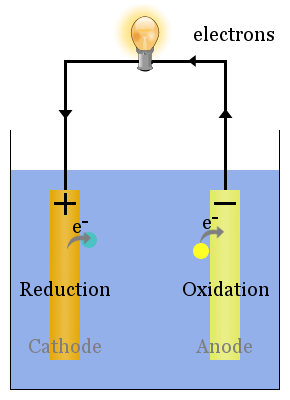
Spontaneous redox reactions at the electrodes produce a voltage. Correctly set up, this voltage can drive electrons through electric devices, such as the lightbulb shown here. In this diagram, species transfer electrons to the anode from where they flow through the lightbulb to the cathode, where they bring about reduction.
Basic Electrolytic
Cell Schematic
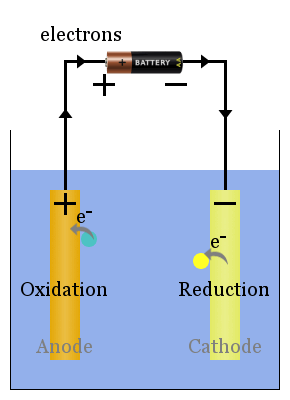
Non-spontaneous redox reactions are driven by an external voltage. The electrolytic cell's processes are the opposite of the voltaic cell's. The current from the power source pushes electrons on to the cathode, where they cause reduction of species to take place.
Example of a Voltaic Cell
The Edison Battery
The Edison battery is a simple, rechargeable cell invented by Thomas Edison. It consists of two metal electrodes, one made of iron, the other of nickel. During initial charging, a coating of nickel oxide forms on the nickel electrode.
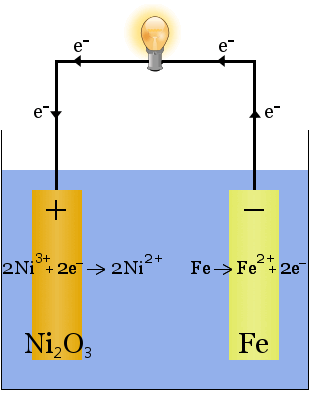
The electrolyte (the ionic liquid between the electrodes) is 20-30 percent by weight potassium hydroxide in water. The potassium hydroxide's role in this case is to increase the ionic conductivity to complete the electric circuit - potassium hydroxide is not consumed in the reaction.
When it is discharging, the Edison cell operates as a voltaic cell. When it is being charged, the cell operates as an electrolytic cell.
The chemical equations for the reactions at the electrodes are:
During discharge, when the cell is delivering electrical energy, the reactions above proceed to the right →.
During charging, when the cell is converting electrical energy to chemical potential energy, the reactions above proceed to the left ←.
The Edison cell, with both electrodes sharing the same electrolyte, is one of the simplest voltaic cells in practical use; most voltaic cells are more complicated. Its great advantages are its remarkable robustness against errors such as overcharging and its very long working life, with almost limitless charge-discharge cycling.
Its greatest disadvantage is that, relative to its energy output, it is very heavy.
Further Example
Lithium Batteries
Advances in battery technology have driven miniaturization of electronic devices. Without these advances, modern devices such as cellphones and tablets would be larger and more cumbersome.
At the heart of these advances has been lithium ion technology. The element lithium is a metal of very low density: at 0.534 g/cm3 lithium is half as dense as water; lithium floats on water. Compare this with the metals in the Edison cell, nickel's density is 8.908 g/cm3, and iron's is 7.874 g/cm3: these are more than 10x higher than lithium's density.
Lithium's high redox potential combined with its low density makes its ions perfect for use as a battery material.
A typical lithium ion battery is based on porous electrodes that allow Li ions to move in and out of their pores. For example, a rechargeable lithium ion battery could have a graphite electrode and a lithium doped cobalt oxide electrode with a polyoxyethylene electrolyte containing the salt LiPF6.
When such a battery is powering a device, lithium atoms held within the layer structure of graphite anode are oxidized to become ions.
At the cathode, lithium ions are reduced within the cobalt oxide structure.
These reactions are reversed when the cell is being charged.
