An electrolyte is a chemical compound that dissociates into ions and hence is capable of transporting electric charge - i.e. an electrolyte is an electric conductor; unlike metals the flow of charge is not a flow of electrons, but is a movement of ions.
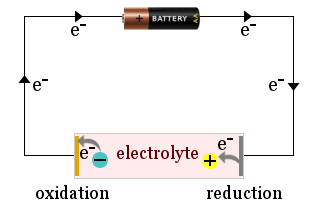
For example, the diagram shows a domestic battery being used to attract electrolyte ions to the electrodes of an electrochemical cell, where the ions gain electrons (are reduced) or lose electrons (are oxidized).
Sometimes the electrodes themselves react, for example a metal electrode could be oxidized and release ions into the electrolyte.
Electrolytes can be solid, liquids, or solutions.
Examples of Electrolytes
1. Molten sodium chloride acts as an electrolyte in the production of sodium metal. Chlorine gas is also produced.
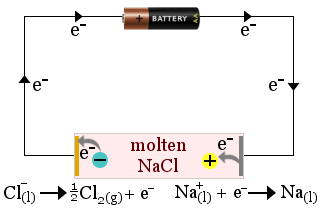 In this case, the electrolyte is consumed by the cell's redox reactions.
In this case, the electrolyte is consumed by the cell's redox reactions.
2. Potassium hydroxide dissolved in water produces a highly conductive electrolyte in the Edison cell, an early rechargeable cell.
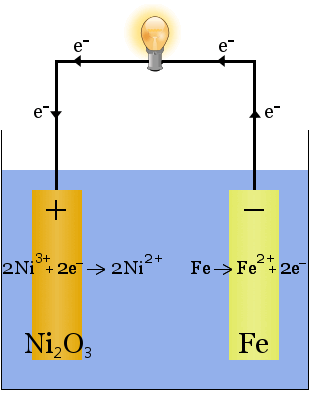
Unlike in the case of sodium production above, no electrolyte is consumed in this reaction. The potassium hydroxide's role in this case is to increase the availability of ions to increase the electrolyte's conductivity and complete the electric circuit.
The chemical equations for the reactions at the electrodes are:
For discharge the equations are read from left to right; for charging right to left.
3. Traditionally electrolytes were liquids to allow movement of ions. However, solid materials are now available that allow ions to move easily through their structures. Solid electrolytes are desirable in many consumer products, because they do not leak.
Lithium tin phosphorous sulfide, Li10SnP2S12, is a solid electrolyte for use in lithium ion batteries. The crystal structure of the solid electrolyte combined with its weak interaction with lithium ions provides an environment that lithium ions can easily hop through.
4. Electrolytes are enormously important for the electrochemical processes in living organisms. The main ions in these electrolytes are calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), chloride (Cl-), hydrogen carbonate (HCO3-), and hydrogen phosphate (HPO42-).
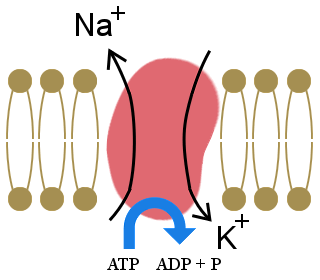
The importance of the sodium-potassium pump, shown in the diagram, to our lives is illustrated by the fact that it uses one-third of our resting energy. The pump maintains our cells' electrolyte balance, with excess potassium ions inside cells and excess sodium ions outside cells.
This concentration gradient creates a voltage across the cell wall, which allows electrical signals to be transmitted in neurons and in muscles. It also provides the energy for processes in cell-membranes.
