Strictly defined, ionization is the complete loss of an electron from an atomic or molecular species. The resulting species is called an ion.

In chemical equations, the charge on ions is shown as a superscript, such as in this simple ionization reaction:
Ions may ionize further:
Positively charged ions are often referred to as cations.
Although in strict terms ionization refers to the formation of a positive ion, in normal usage, the word also includes the formation of a negative ion:
Negatively charged ions are often referred to as anions.
Direct Ionization of Elements
Metals typically form cations and non-metals typically form anions.
Some elements, such as carbon, gold, and the noble gases, do not readily form ions.
The alkali metals in Group 1 of the periodic table and the halides in Group 17 ionize very readily. Alkali metals need only lose one electron to obtain a full electron shell: likewise, halides need only gain one electron to achieve this. For example, sodium and chlorine react spontaneously by ionizing to form the ionic compound sodium chloride:
Potassium and water react by ionizing to produce the ionic compound potassium hydroxide plus hydrogen:
Ionization of Molecules in Solution
Hydrogen chloride gas molecules readily ionize in water to produce hydrochloric acid.
Self-Ionization
In water, equilibrium exists between water molecules and ions resulting from water's self-ionization. (See amphiprotic.)
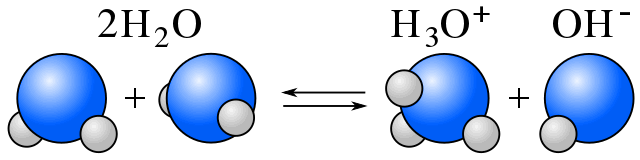
Ionization in Plasma
At very high temperatures, electrons are stripped from atoms to form a plasma.
For example, in the sun's corona:
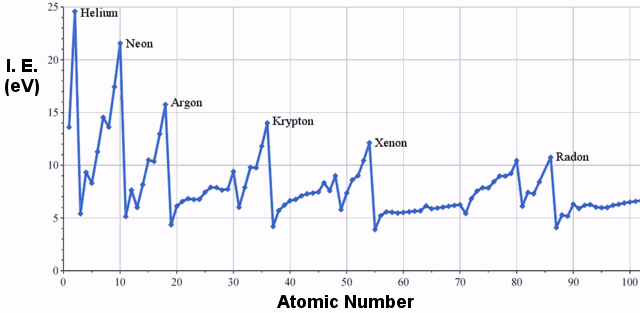
Examples of Ionization Energy
The first ionization energies of elements in the third row of the periodic table are as follows:
First Ionization Energy
| Element | First Ionization Energy (eV) |
|---|---|
| Sodium | 5.14 |
| Magnesium | 7.65 |
| Aluminum | 5.99 |
| Silicon | 8.15 |
| Phosphorus | 10.49 |
| Sulfur | 10.36 |
| Chlorine | 12.97 |
| Argon | 15.76 |
Clearly, there is a trend of increasing ionization energy moving from left to right in the periodic table. The graph below illustrates this trend for a wider selection of elements: