As you might expect from the name, superacids are extremely strong acids, much stronger than traditional strong mineral acids such as sulfuric acid and hydrochloric acid.
Acidity refers to the ability to add protons, H+, to other substances: the stronger the acid, the greater its power to protonate other substances.
A superacid is defined as a substance that has a higher H+ chemical potential than 100% pure sulfuric acid.

List of Superacids
| Substance | Formula | H0 | Comments |
|---|---|---|---|
| Chlorosulfuric acid | HSO3Cl 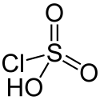 |
-12.8 | Reacts with alcohols to produce alkyl sulfate detergents. |
| Triflic acid | CF3SO3H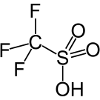 |
-14.1 | Also known as trifluoromethanesulfonic acid, or TFMS. Reacts with alcohols to produce ethers and olefins. |
| Oleum | H2SO4 + SO3 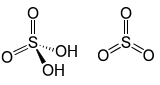 |
-15 | Sulfur trioxide dissolved in pure sulfuric acid produces oleum, which is a preferred method, via dilution with water, of producing concentrated sulfuric acid. Oleum is also used as a reactant in manufacturing explosives such as nitrocellulose. |
| Fluorosulfuric acid | HSO3F 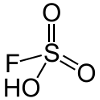 |
-15.1 | Is used to prepare magic acid (see below) and for regenerating the HF and H2SO4 mixtures used to etch lead glass. |
| Carborane acid | H(CHB11X11) |
-18.0 | The carborane shown on the left is fluorinated; chlorinated carboranes are also superacids, but with lower acidity. Fluorinated carborane acid is the only acid capable of protonating CO2 to produce [H(CO2)2]+. Carborane is the only acid capable of protonating C60 without side reactions. Carborane protonates benzene to yield C6H7+ salt. Although they have great protonating power, carboranes are less corrosive that other superacids; unlike most, they can be stored in a glass bottle. |
| Magic acid | FSO3H·SbF5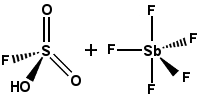 |
-19.2 | Prepared from fluorosulfuric acid (see above) and antimony pentafluoride. Catalyzes the isomerization of saturated hydrocarbons, stabilizes carbocations. |
| Fluoroantimonic acid | H2FSbF6 or H2F[SbF6]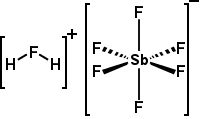 |
-31.3 | The most powerful superacid, it is produced by reacting a 2:1 mole ratio of HF with SbF5. Can protonate almost any organic compound. |
Acidity is indicated by the Hammett acidity function, H0.
Like pH, H0 is a measure of acidity; lower values of H0 mean higher protonating power. Like pH, H0 is a logarithmic scale.
100% sulfuric acid has an H0 of -12. Compare this with fluoroantimonic acid's H0 of -31. This means fluoroantimonic acid is 1019 more acidic than pure sulfuric acid. That's a bigger difference than there is between the size of the sun and the size of an amoeba.
