When hydrogen is covalently bonded to a highly electronegative atom, such as fluorine, chlorine, oxygen, or nitrogen, the H atom has a partial positive charge, written Hδ+.
Hδ+ is physically very small, so the density of charge on it is unusually high.
Imagine another negative or electronegative atom, say on a different molecule, approaches the Hδ+; there will be mutual attraction, resulting in a particularly strong dipole-dipole attraction. This attraction is called a hydrogen bond.
In general, hydrogen bonds are weaker than ionic and covalent bonds, but are stronger than van der Waals forces.

Hydrogen Bonding in Water
The best known example of hydrogen bonding is water:
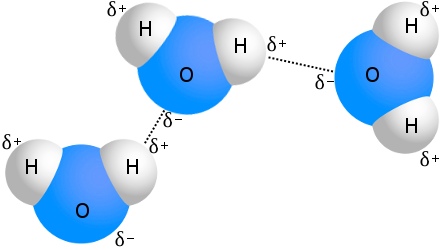
Every water molecule can be hydrogen bonded to as many as four other water molecules. In water at room temperature, the average number of hydrogen bonds per water molecule is 3.6.
The random thermal movement of molecules ensures that the lifetime of any individual hydrogen bond in water is short, averaging only 10 picoseconds. However, the time to form a new bond is even shorter.
To permanently break a single hydrogen bond in water takes 21 kJ mol-1, a significant input of energy. The result is that water's melting and boiling points are much higher than would be expected for such a low molecular weight molecucle.
Consider water vs methane. Water molecules are rather strongly attracted to one another by hydrogen bonding, while van der Waals forces prevail in methane. So, although their molecular masses are similar, at 18 for water and 16 for methane, their physical properties are very different. For example, water melts at 0.00 °C and boils at 99.98 °C; methane melts at -182.5 °C and boils at -161.5 °C.
Hydrogen Bonding in
Water vs Hydrogen Sulfide
Sulfur is in group 16 of the periodic table, the same as oxygen. Sulfur is heavier than oxygen, so H2S should have higher melting and boiling points than H2O. However, this is not the case because sulfur is less electronegative than oxygen, and therefore hydrogen bonding in H2S is weak.

Mol. Mass: 16
Melt Pt.: 0.00 °C
Boil Pt.: 99.98 °C
Electronegativity of O (Pauling): 3.44
hydrogen bonding enthalpy: 21 kJ mol-1

Mol. Mass: 34
Melt Pt.: -82 °C
Boil Pt.: -60 °C
Electronegativity of S (Pauling): 2.58
hydrogen bonding enthalpy: ≅ 3 kJ mol-1
Hydrogen Bonding
in Ammonia and Hydrogen Fluoride
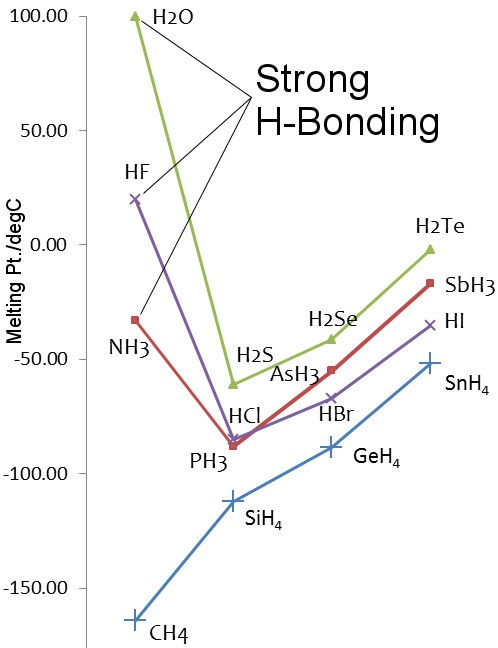
Fluorine and nitrogen are the most electronegative elements in their periodic table groups, and hydrogen bonding is observed in hydrogen fluoride and ammonia.
As in the case of water, hydrogen fluoride and ammonia's melting and boiling points are higher than the hydrides of heavier elements in their groups.
Melting Points for the Hydrides of Group 14-17 Elements
Hydrogen Bonding in DNA
DNA's base pairs link its two helix chains. The base pairs carry DNA's genetic information.
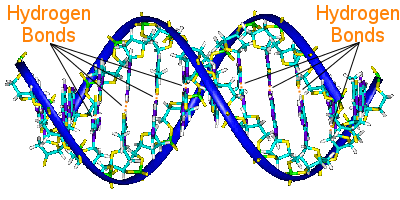
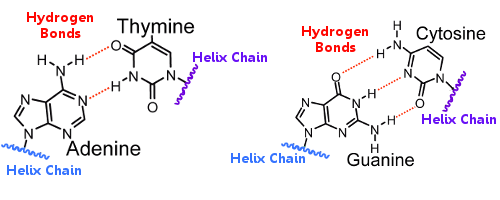
The base pairs on one helix are linked to the base pairs on the other helix by hydrogen bonds. Thus hydrogen bonding holds the helixes together, creating the famous double helix.
When DNA replicates, the hydrogen bonds break, allowing the two helixes to separate. In this way, hydrogen bonding plays an essential role in the base pair lock-and-key mechanism of DNA replication.
Hydrogen Bonding in Wool
Like other protein fibers, wool features hydrogen bonding. The image below shows how hydrogen bonds (orange dots) link the coils of wool's α-helix chain (green).
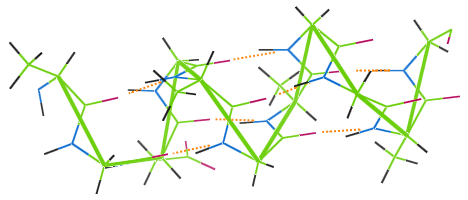
If a woolen garment is washed at a high temperature, the hydrogen bonds are destroyed, the coils lose their elasticity, and the garment becomes mishapen.
