Coulometry is an analytical method for measuring an unknown concentration of an analyte in solution by completely converting the analyte from one oxidation state to another. Coulometry is an absolute measurement similar to gravimetry or titration and requires no chemical standards or calibration. It is therefore valuable for making absolute concentration determinations of standards.
Coumetry uses a constant current source to deliver a measured amount of charge. One mole of electrons is equal to 96,485 coulombs of charge, and is called a faraday.
Schematic of a coulometric cell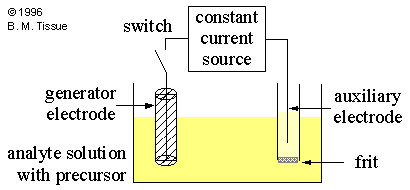 Coulometric Titration
Coulometric Titration
Due to concentration polarization it is very difficult to completely oxidize or reduce a chemical species at an electrode. Coulometry is therefore usually done with an intermediate reagent that quantitatively reacts with the analyte. The intermediate reagent is electrochemically generated from an excess of a precursor so that concentration polarization does not occur. An example is the electrochemical oxidation of I- (the precursor) to I2 (the intermediate reagent). I2 can then be used to chemically oxidize organic species such as ascorbic acid.
The point at which all of the analyte has been converted to the new oxidation state is called the endpoint and is determined by some type of indicator that is also present in the solution. For the coulometric titration of ascorbic acid, starch is used as the indicator. At the endpoint, I2 remains in solution and binds with the starch to form a dark purple complex.
The analyte concentration is calculated from the reaction stoichiometry and the amount of charge that was required to produce enough reagent to react with all of the analyte.
