A complex is a molecular entity formed by loose association involving two or more component molecular entities (ionic or uncharged), or the corresponding chemical species.

The bonding between the components is normally weaker than in a covalent bond.
In inorganic chemistry the term "coordination entity" is recommended instead of "complex" [IUPAC inorganic NOMENCLATURE (1990)].
Commonly used Definition of 'Complex'
Despite IUPAC's recommendation, we must remember that many chemists continue to use the word "complex" to indicate something that consists of a metal atom or ion in the center, surrounded by ligands.
i.e. a metal center plus ligands is commonly called a complex. Complexes can be positively charged, neutral, or negatively charged. The overall charge on the complex depends on the oxidation state of the metal and the charges brought by the ligands.
Example 1:
If a complex forms between an Fe2+ ion and six CN- ligands, the complex will have a -4 charge, and the formula is written [Fe(CN)6] 4-.
Example 2:
If a complex forms between an Ag+ ion and two NH3 ligands, the complex will have a +1 charge, and the formula is written [Ag(NH3)2] +.
For a different usage of "complex" in inorganic chemistry, see IUPAC Inorganic RULES (1970); Rule 2.24. See also activated complex, adduct, charge transfer complex, electron-donor-acceptor complex, encounter complex, inclusion complex, s-adduct, p-adduct, transition state.
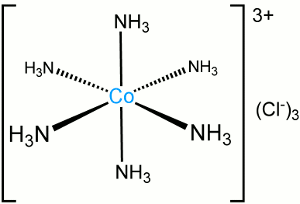
Example of a Complex
The image shows a cobalt (III) complex (or coordination entity). The central metal ion has six ammonia molecules attached - these attached molecules are called ligands.
The complex forms because the lone pairs of electrons on the ammonia ligands are attracted to the positive charge on the cobalt ion. The bond between a ligand and the central metal is weaker than a typical covalent or ionic bond. Another way of saying this is that a complex forms when a lewis acid (the metal in the center) and lewis base(s) (the ligands) combine to form a coordination compound.
The complex has the same charge as the central cobalt, because the ammonia ligands are neutral. The three chloride ions are not ligands, they are normal chloride ions; they form ionic bonds with the cobalt complex.
d Electrons Control the Properties of Transition Metal Complexes
Complexes are formed when ligands add to transition metals (d-block metals): metals such as titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, gold, platinum, palladium, etc.
The number of d electrons on the central metal is crucial. Transitions metals can exist in different oxidation states - i.e. with different numbers of d electrons.
By oxidizing or reducing the metal in the center of a complex, with the number and type of ligands unchanged, many of a complex's properties will change, such as: stereochemistry, stability, spectroscopic, magnetic and reactivity.
Non-Transition Metal Complexes
Other metals, such as magnesium, aluminum, calcium and tin also form complexes with suitable ligands. For example Al[H2O]63+.
Another Example of a Complex
In the first example above, there were ammonia ligands around a central cobalt ion. Chloride ions were the counterions.
Negatively charged species like chloride are electron donors, so it's possible to find them in complexes as ligands.
An example is the tetrachlorocobaltate complex anion, whose formula is [CoCl4]2- (see image). The cobalt is in oxidation state 2, binding with four chloride ions. Each one of the chloride ions brings one negative charge to the complex, so the total charge on the complex is -2. It is a complex anion.