In a titration, a solution of unknown concentration is reacted with a solution of a known concentration in order to find out more about the unknown solution, usually its concentration.
Two of the most common titrations are acid-base and redox titrations.
Acid-Base Titrations
In an acid-base titration one of the solutions is an acid and the other a base.
One is placed in a flask. The other is placed in a burette, from which it is dripped into the flask until the titration reaches its end point.
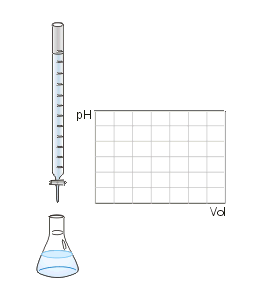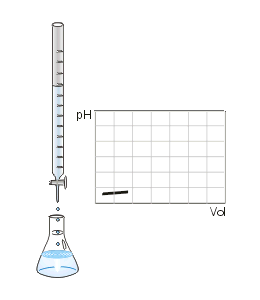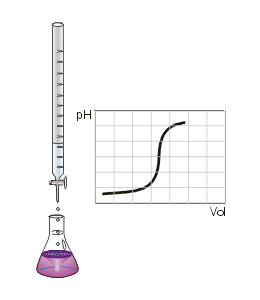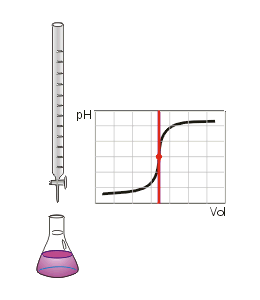
A suitable indicator needs to be chosen such that the end point shows accurately that all of the solution in the flask has reacted with the solution being dripped into it - the point at which this happens is called the equivalence point.
In a perfect titration the end point and equivalence point will be identical. If the indicator is not chosen well, the end and equivalence points will differ, and the titration will not produce accurate data about the solution of interest.
Redox Titrations
In a redox titration one of the solutions is a reducing agent and the other an oxidizing agent.
The equivalence point is reached after sufficient oxidizing agent has been added to react with all of the reducing agent.
A redox indicator, which changes color between reduced and oxidized states, can be used to detect the end point.
