The wavelengths of X-rays are of the same order of magnitude as the distances between atoms or ions in a molecule or crystal (10-10 m, which equals 1 Å).
A crystal diffracts an X-ray beam passing through it to produce beams at specific angles depending on the X-ray wavelength, the crystal orientation, and the structure of the crystal.
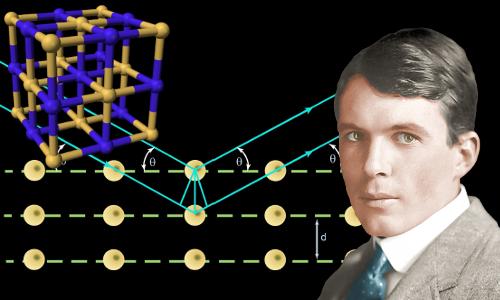
X-rays are predominantly diffracted by electron density and analysis of the diffraction angles produces an electron density map of the crystal. Since hydrogen atoms have very little electron density, determining their positions requires extensive refinement of the diffraction pattern.
Electron diffraction and neutron diffraction are sensitive to nuclei and are often used to accurately determine hydrogen positions. Instrumentation
X-ray diffractometers consist of an X-ray generator, a goniometer and sample holder, and an X-ray detector such as photographic film or a movable proportional counter. X-ray tubes generate X-rays by bombarding a metal target with high-energy (10 - 100 keV) electrons that knock out core electrons.
An electron in an outer shell fills the hole in the inner shell and emits an X-ray photon.
Two common targets are Mo and Cu, which have strong K(alpha) X-ray emission at 0.71073 and 1.5418 Å respectively. X-rays can also be generated by decelerating electrons in a target or a synchrotron ring. These sources produce a continuous spectrum of X-rays and require a crystal monochromator to select a single wavelength.

Related topics:
- Electron diffraction
- Neutron diffraction
- Powder X-ray diffraction
