There are different types of chemical reactions. The first thing to ask yourself when thinking about mixing reactants is: What could happen? In the following examples the subscripts in the reactions refer to pure solids: AgCl(s), liquids: H2O(l), gases: H2 (g), or aqueous solution: Cl-(aq). What is a Chemical Reaction?
A chemical reaction occurs when substances (the reactants) collide with enough energy to rearrange to form different compounds (the products). The change in energy that occurs when a reaction take place is described by thermodynamics and the rate or speed at which a reaction occurs is described by kinetics. Reactions in which the reactants and products coexist are considered to be in equilibrium. Gas-Phase Reactions
Gases can react with other gases, liquids, or solids to form new products. Some examples are:
Combustion of hydrogen gas: 2 H2 (g) + O2 (g) ![]() 2 H2 (l)
2 H2 (l)
Combustion of methane: CH4 (g) + O2 (g) ![]() CO2 (g) + 2 H2O(l)
CO2 (g) + 2 H2O(l)
Heating calcium carbonate (limestone) to make calcium oxide (lime): CaCO3 (s) + heat ![]() CaO(s) + CO2 (g)Acid-Base Reactions
CaO(s) + CO2 (g)Acid-Base Reactions
The Bronsted-Lowry definition describes acids as proton donors and bases as proton acceptors. Acids and bases can react with water and also react together. An acid in water will dissociate and a base will undergo hydrolysis, which means that it splits a water molecule. The acid dissociation and base hydrolysis reactions are described in the acid-base equilibrium document.
The reaction of an acid and a base is called a neutralization reaction. Mixing acids and bases results in neutralization because the base will accept the proton that the acid donates. What remains when an acid and base react depends on the relative amounts of the acid and base. The following example shows neutralization of equal molar quantities of a strong acid and a strong base.
HNO3 + NaOH ![]() H2O(l) + Na+(aq) + NO3-(aq)
H2O(l) + Na+(aq) + NO3-(aq)
The spectator ions are usually left out of the reaction and the "net" reaction is:
H+(aq) + OH-(aq) ![]() H2O(l)Precipitation Reactions
H2O(l)Precipitation Reactions
Many compounds have limited solubility in aqueous (water) solution. When the concentrations of the ions in solution rise above the solubility limit, the ions combine to form solid particles that precipitate from solution. The concentrations of the ions remaining in solution are governed by the equilibrium constant, Ksp, which is called the solubility product.
Example: When chloride is added to a silver solution, solid silver chloride precipitates from solution. The resulting equilibrium is always written in the direction of the solid dissolving:
AgCl(s) 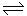 Ag+(aq) + Cl-(s)Complexation Reactions
Ag+(aq) + Cl-(s)Complexation Reactions
Metal ions in solution can bind with ligands to form soluble complexes. These reactions are always described by the equilibrium expression written in the direction of complex formation. The equilibrium constant, Kf, is called the formation constant .
Example: When ethylenediaminetetraacetic acid (EDTA) is added to a solution containing calcium ion a calcium edta complex forms in solution:
Ca2+(aq) + EDTA4-(aq) 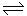 Ca(EDTA)2-(aq)Redox Reactions
Ca(EDTA)2-(aq)Redox Reactions
Reduction-oxidation (redox) reactions involve the transfer of electrons from one species to another. One specie will be oxidized and the other will be reduced.
Example: Zn(s) + Cu2+(aq) ![]() Zn2+(aq) + Cu(s)
Zn2+(aq) + Cu(s)
In this reaction two electrons are transferred from each zinc atom to each copper ion. The zinc metal is oxidized to zinc ions and the copper ions are reduced to copper metal.
