The nucleus (plural nuclei) is the central part of the atom; it contains the protons and neutrons.

Nearly all of the atom's mass is contained in the nucleus; the volume of the nucleus is tiny compared with the atom's total volume; thus the density of matter in the nucleus is enormous.
Average nuclear density is 2.3 x 1017 kg/m3.
If a human of mass 70 kg took up the same amount of space as a sphere containing only his/her atomic nuclei, the sphere's radius would be only about 0.0004 cm.
All nuclei are positively charged because of the presence of protons. Nearly all nuclei contain both protons and neutrons: the exception is the most fundamental and smallest of all atoms, hydrogen-1, whose nucleus consists of a single proton.
The number of protons determines the chemical element. Thus an element with one proton must be hydrogen, an element with two protons must be helium, an element with three protons must be lithium,... etc.
The number of neutrons contained in the nucleus of most elements is variable. Nuclei with the same number of protons and different numbers of neutrons are called isotopes.
The number of protons a nucleus has is given by the element's atomic number, while the number of protons plus neutrons is given by an element's mass number. This is usually shown in the following way:
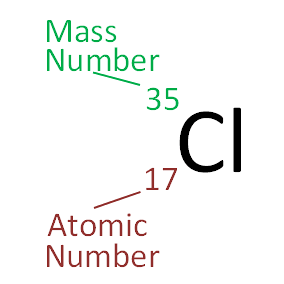
The atomic nucleus has little influence on the chemistry of an element. So, although, for example, the nuclei of carbon-12 and carbon-14 are fundamentally different in that the first is stable and the second is second radioactive, their chemical reactions are essentially identical.