Recall that titration is the quantitative measurement of an analyte in solution by reacting it completely with a standardized reagent.
Complexes form in a fixed stoichiometry so a standard solution of a ligand can be used to titrate a metal ion in solution.
Similarly, a standard solution of a metal ion can serve as the titrant for a species that acts as a ligand.
Ethylenediaminetetraacetic acid (EDTA) is a common chelate because it makes 6 bonds with metal ions to form 1:1 complexes with large formation constants. The fully protonated form of EDTA is:
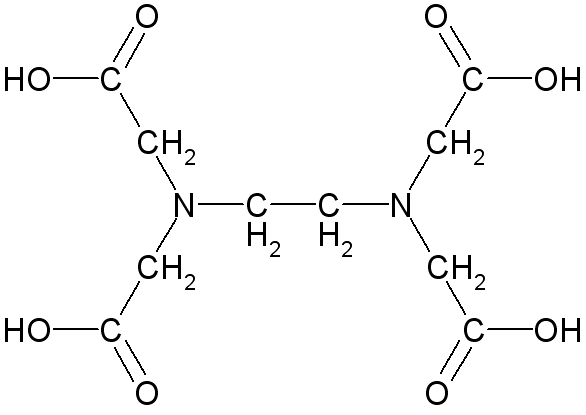
The two nitrogen atoms can donate their lone pairs to form two bonds and the four -OH groups can lose their protons to form four more bonds to the metal.
Kf Values for Some EDTA Complexes
Metal Name Kf
Ag +silver 2.1 x 107
Al 3+ aluminum 1.3 x 1016
Ba 2+ barium 5.8 x 107
Ca 2+ calcium 5.0 x 1010
Cd 2+ cadmium 2.9 x 1016
Co 2+ cobalt 2.0 x 1016
Fe 2+ iron(II) 2.1 x 1014
Fe 3+ iron(III)1 x 1025
Hg 2+ mercury 6 x 1021
Ni 2+ nickel 4.2 x 1018
Pb 2+ lead 1.1 x 1018
Zn 2+ zinc 3.2 x 1016
Calmagite Indicator for EDTA Titrations
Calmagite indicator has two -OH groups with acidic protons. The color of calmagite changes depending on whether or not these protons are present. At pH=10 one proton is present and the color of the indicator is blue. A calcium or magnesium ion can displace both protons to form a calmagite-metal complex, which has a red color. Ca2+ and Mg2+ can be titrated using EDTA as the titrant and calmagite indicator because the EDTA binds Ca2+ and Mg2+ more strongly than the indicator. At the endpoint, the EDTA will bind all of the metal, leaving the calmagite with no metal ions. A solution containing calmagite will turn from red (or purple very near the endpoint) to blue.
