Two molecules are described as stereoisomers if they are made of the same atoms connected in the same sequence, but the atoms are positioned differently in space.
The difference between stereoisomers can only be seen when the three-dimensional arrangement of the molecules is considered.
Stereoisomers can be subdivided into optical isomers and geometric isomers.

Optical isomers are molecules which are mirror images of one another. Often these mirror image molecules are referred to as enantiomers.
Just as a right-handed glove cannot be superimposed on a left-handed glove, optical isomers cannot be superimposed on one another.
One molecule is a mirror image of the other
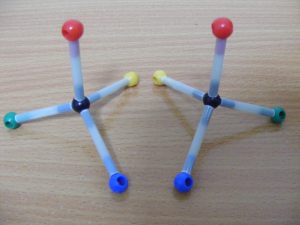
Mirror image configurations of bromo-chloro-fluoro-methane
No matter how we turn these molecules, we cannot arrange them so the atoms are identically oriented in space. Mirror image configurations cannot be superimposed.
Optical Isomers Cannot Be Superimposed
 Mirror image configurations of bromo-chloro-fluoro-methane
Mirror image configurations of bromo-chloro-fluoro-methane
Optical isomers can be described as left- or right-handed. Naturally occurring amino acids, the building blocks of life, are nearly all found in the left-handed state and produce left-handed proteins.
When people refer to geometric isomers they are usually referring to cis-trans isomers. Cis-trans isomers can be found when rotation around a chemical bond is impossible, as seen in molecules with double and triple bonds.
For example cis-butenedioic acid and trans-butenedioic acid are stereoisomers - specifically geometric isomers.
As you can see in the image below, in the cis-isomer, both of the acid groups lie on the same side of the double bond. In the trans-isomer, the acid groups are on opposite sides of the double bond.
Cis-Trans Geometric Isomers
 Cis and Trans Isomers of Butenedioic Acid
Cis and Trans Isomers of Butenedioic Acid
In contrast to the stereoisomers discussed above, structural isomers are not stereoisomers.
They arise when compounds have the same molecular formula but the atoms are bonded together in different sequences.
For example chloropropane can exist in two forms:
1-chloropropane: CH3CH2CH2Cl and 2-chloropropane: CH3CHClCH3
In 1-chloropropane the chlorine is bonded to an end carbon while in 2-chloropropane the chlorine is bonded to the middle carbon.

