A radical is a chemical species that contains an unpaired electron. In the past such species were often called "free radicals".

Radicals are usually formed when a single covalent bond breaks to leave an unpaired electron on each of the two species created by the bond breaking: this is called homolysis.
Homolysis is shown in the diagram below, where a single electron moves from the bond to each atom, producing radicals A. and B..
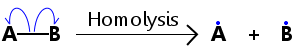
Dots are placed on radical species to show that they possess a single, unpaired electron.
Cleaving a chemical bond requires energy to be input, such as heat or light. In general, radicals are highly reactive and form new bonds again very quickly.
A radical may be electrically neutral, positively charged (radical cation) or negatively charged (radical anion).
1. The chlorine molecule Cl2 undergoes homolysis in ultraviolet light to form two Cl. radicals. These are electrically neutral and highly reactive.
2. Bromine water is used in organic chemistry as a test to detect alkenes. Bromine water is brown-orange. When added to alkenes, it decolorizes. The reason for this is that the brown-orange bromine dissolved in water undergoes homolysis and reacts with alkenes. This is an example of an addition reaction.
3. When tobacco is burned to produce cigarette smoke, the smoke contains a large number of free radical molecules. These free radicals are highly reactive, and when tobacco smoke is inhaled, react with lung tissues. This results in inactivation of alpha 1-antitrypsin, promoting the development of the fatal lung disease emphysema.