What are Ionic Compounds?
Ionic compounds are compounds consisting of ions.
Two-element compounds are usually ionic when one element is a metal and the other is a non-metal. Examples include:
- sodium chloride: NaCl, with Na+ and Cl- ions
- lithium nitride: Li3N, with Li+ and N3- ions
- magnesium oxide: MgO, with Mg2+ and O2- ions
- calcium phosphide: Ca3P2, with Ca2+ and P3- ions
Ionic compounds can be more complicated than the two-element ones listed above. Examples of polyatomic ionic compounds include:
- sodium sulfate: Na2SO4, with Na+ and SO42- ions
- ammonium thiocyanate: NH4SCN, with NH4+ and SCN- ions
- potassium hydroxide: KOH, with K+ and OH- ions
- ammonium carbonate: (NH4)2CO3, with NH4+ and CO32- ions
Although they consist of positively and negatively charged ions, ionic compounds are electrically neutral, because the charges are always equal and opposite.
(The other major group of compounds is molecular compounds: these consist of molecules rather than ions.)
Bonding in Ionic Compounds
In its purest form, ionic bonding is not directional. It can be regarded as simple positive-negative Coulombic attraction between point charges. This is different from covalent bonding, in which electrons are shared by atoms, forming directional bonds.
However, absolutely pure ionic bonding does not exist. There is always at least a small degree of covalent bonding character in ionic compounds.
The Formulas and Structures of Ionic Compounds
In a molecular compound, such as, for example, water, H2O, or ethanol, C2H5OH, each unit of the substance, the molecule, consists of the number of atoms shown in the formula.
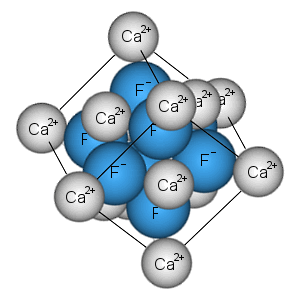
In an ionic compound, such as common salt, NaCl, or magnesia MgO, the formula tells us the correct ratio of elements present, but it does not specify the unit. Ionic compounds exist as giant crystal lattices.
Each crystal contains an unspecified number of ions: the numbers are enormous: even a 0.5 gram crystal of sodium chloride contains about 1 x 1022 ions.
The structures of crystals can be understood in terms of their repeating units - unit cells: these can be determined by X-ray diffraction measurements.

Image by Crystal Titan.

Electric Conductivity
In general, ionic solids do not carry electric current, because the ions are in fixed positions in the crystal lattice. They do, however, conduct electricity when molten or when dissolved in solution, when the ions are released from the crystal lattice and become mobile.
Solutions of Ionic Compounds
Ionic compounds dissolve best in polar solvents. Examples of polar solvents are water, methanol, and formamide.
Ionic compounds are insoluble or have very low solubility in non-polar solvents. Examples of non-polar solvents are hydrocarbons, and chloroform.
Solvation
For an ionic compound to dissolve, the electrostatic bonds holding the ions in their rigid crystal lattice must be replaced by attractions between ions and solvent molecules.
Each ion becomes surrounded by solvent molecules, as shown in the diagram.
Solvation of ionic compounds in polar solvents is driven by a decrease in free energy.
Na+ Solvation Shell
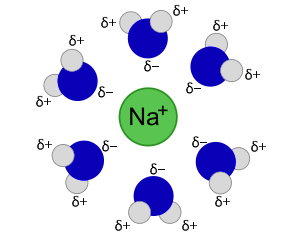
When NaCl dissolves in water, the polar water molecules form solvation shells around the dissolved ions. Here, water's oxygen atoms, each with a slight negative charge, solvate the Na+
Cl- Solvation Shell
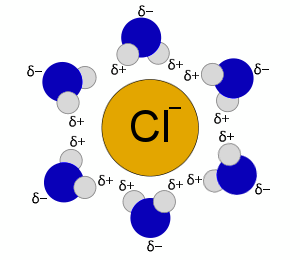
Likewise, the Cl- is surrounded by water molecules with their slightly positive hydrogen atoms oriented to the Cl-.
Acids and Bases
Bronsted Lowry acids/bases are substances that can donate/accept H+ ions.
Substances containing H+ ions are classed as acids.
H+ acceptors such as OH- and O2- are classed as bases.
