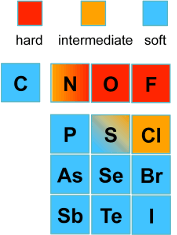
What are Hard and Soft Acids and Bases?
The HSAB concept's intention is to identify the products of Lewis acid-base reactions that have the greatest stability.
In considering the acid-base equilibrium:

it was found that the right side is favored when the Lewis acids and bases are only weakly polarizable: in other words, their electron shells are not easily deformed.
This led to the hard and soft acid and base (HSAB) concept. Other things being approximately equal:
- hard acids react faster with hard bases and form stronger bonds with them
- soft acids react faster with soft bases and form stronger bonds with them
HSAB is a qualitative guide to reactions; it is not a quantitative, numerical method.
Hard Acids and Bases
Hard acids consist of small highly charged cations and molecules in which a high positive charge can be induced on the central atom.
Examples of Hard Acids: H+, Li+, K+, Ca2+, Al3+, Sn4+, BF3, BCl3, CO2, RCO+, SO3, RMgX, VO2+, AlCl3
Hard bases are highly electronegative and of low polarizability.
Examples of Hard Bases: F-, OH-, NH3, N2H4, ROH, H2O, SO42-, PO43-
Hard bases react more readily to form stable compounds and complexes with hard acids.
Soft Acids and Bases
Soft acids consist of large low charge cations and molecules with relatively high energy occupied molecular orbitals. Soft acids are readily polarizable.
Examples of Soft Acids: Cs+, Cu+, Au+, Pt2+, Hg+, BH3, Br2, I2, RO+, quinones
Hard bases have low electronegative and low polarizability.
Examples of Soft Bases: H-, R-, CO, PR3, C6H6, SCN-
Soft bases react more readily and form stable compounds and complexes with soft acids.
Click link for Further Examples of Hard and Soft Acids and Bases
Hard - Soft Acid Ions

Hard - Soft Base Ions