A catalyst is a substance that speeds up a chemical reaction, but is not consumed by the reaction; hence a catalyst can be recovered chemically unchanged at the end of the reaction it has been used to speed up, or catalyze.

Discussion
For chemicals to react, their bonds must be rearranged, because the bonds in the products are different from those in the reactants. The slowest step in the bond rearrangement produces what is termed a transition state - a chemical species that is neither a reactant nor a product, but is an intermediate between the two.
Energy is required to form the transition state. This energy is called the activation energy, or Ea. Reading the diagram below from left to right shows the progress of a reaction as reactants pass through the transition state to become products.
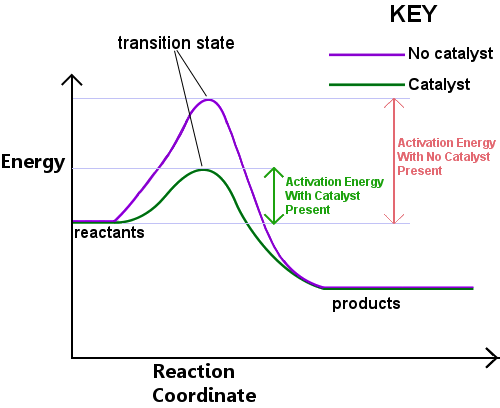
The activation energy can be thought of as a barrier to a chemical reaction, a hurdle that must be crossed. If the barrier is high, few molecules have sufficient kinetic energy to collide, form a transition state, and cross the barrier. Reactants with energy lower than Ea cannot pass through the transition state to react and become products.
A catalyst works by providing a different route, with lower Ea, for the reaction. Catalysts lower the energy barrier. The different route allows the bond rearrangements needed to convert reactants to products to take place more easily, with a lower energy input. In any given time interval, the presence of a catalyst allows a greater proportion of the reactant species to pick up sufficient energy to pass through the transition state and become products.
Example 1: The Haber Process
The Haber process, which is used to make ammonia from hydrogen and nitrogen, is catalyzed by iron, which provides atomic sites on which the reactant bonds can rearrange more easily to form the transition state.
Example 2: Enzymes
In our bodies, and in other living things, enzymes are used to speed up biochemical reactions. An enzyme is a type of catalyst.
Complex life would be impossible without enzymes to allow reactions to take place at suitable speeds.
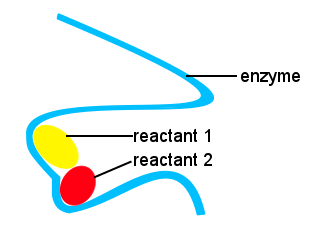
The shapes of enzymes along with locations on the enzyme that bind to the reactants provide an alternative reaction pathway, allowing specific molecules to come together to form a transition state with a reduced activation energy barrier.
In the schematic below, the long chain enzyme provides sites for reactant molecules to come together to form a transition state with a low activation energy.
Catalysts cannot shift the position of a chemical equilibrium - the forward and backward reactions are both accelerated so that the equilibrium constant Keq is unchanged. However, by removing products from the reaction mixture as they form, the overall rate of product formation can in practice be increased.