Cyclohexane can exist in various conformations; the 'chair' (see image below) is energetically the most stable of these.
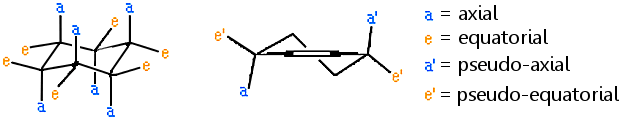
Each carbon atom in the cyclohexane ring makes four bonds. Two of these bonds are to other ring carbon atoms, and two bonds are made to non-ring atoms. The bonds to non-ring atoms are termed axial or equatorial, depending on the bond angle.
- Bonds to non-ring atoms with angles of about 90° to the ring plane are termed axial.
- Bonds to non-ring atoms which make only a small angle compared with the plane of the ring are termed equatorial.
Thus the axial bonds are approximately parallel to the C3 axis and the equatorial bonds approximately parallel to two of the ring bonds.
These terms are also used for the chair form of other saturated six-membered rings. The corresponding bonds occurring at the allylic positions in mono-unsaturated six-membered rings are termed pseudo-axial (or quasi-axial) and pseudo-equatorial (or quasi-equatorial). The terms axial and equatorial have similarly been used in relation to the puckered conformation of cyclobutane, crown conformer of cyclooctane, etc. and the terms pseudo-axial and pseudo-equatorial in the context of the non-planar structures of cyclopentane and cycloheptane. (See apical, basal, equatorial for an alternative use of axial and equatorial with bipyramidal structures.)

