Electronegativity is a measure of how strongly atoms attract bonding electrons to themselves.
Its symbol is the Greek letter chi: χ
The higher the electronegativity, the greater an atom's attraction for electrons.
Below is a periodic table of electronegativity: the lighter the shade of green, the higher the electronegativity. Gray means no value is known. (Click image for larger view that shows electronegativity values.)
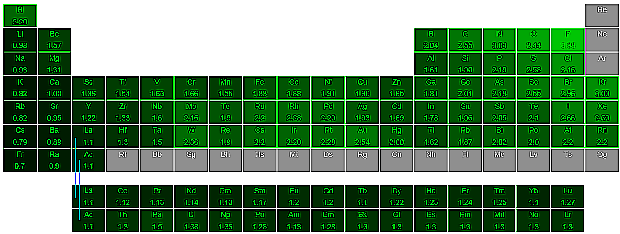

Looking at the elements in the periodic table, in general, electronegativity of elements increases from:
- the bottom of a group to the top (although this does not apply to the transition metals) and
- left to right across a period
Negative Ions
The most electronegative element is fluorine, followed by oxygen, chlorine and nitrogen.
Atoms with high electronegativity tend to form negative ions.
Positive Ions
The least electronegative (most electropositive) element is cesium, followed by rubidium, potassium and barium. Highly electropositive elements have a strong tendency to form positive ions.
Ionic Compounds
Compounds formed between elements with widely differing electronegativities tend to be ionic compounds, with ionic bonding. Examples of ionic compounds are potassium fluoride, lithium nitride, and magnesium oxide.Covalency
When atoms of similar electronegativity react, they tend to produce covalent bonds in which electrons are shared between atoms. Examples are C-C bonds and C-H bonds.
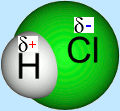
Polar Covalency
When a higher electronegativity atom is covalently bonded to an atom of lower electronegativity, the greater share of the bonding electrons is taken by the higher electronegativity atom.
The result of this uneven sharing of electrons is a small negative charge on the more electronegative atom and a small positive charge on the atom it is bonded to. This is shown in the HCl example. Such a bond is described as a polar covalent bond.
Electronegativity Scales
Electronegativity is a relative scale - it is calculated rather than measured. Various scales of electronegativity have been devised - for example the Pauling Scale.
Linus Pauling assigned fluorine's electronegativity as 4, and then calculated the electronegativities of other elements relative to this number using bond energies. Fluorine is now assigned an electronegativity of 3.98, because this value gives the best internal consistency when calculations are performed over a wide range of compounds.
Another scale is the Mulliken scale, which bases electronegativity values on the following equation:
Electronegativity = 0.5 x (Ionization Potential + Electron Affinity)